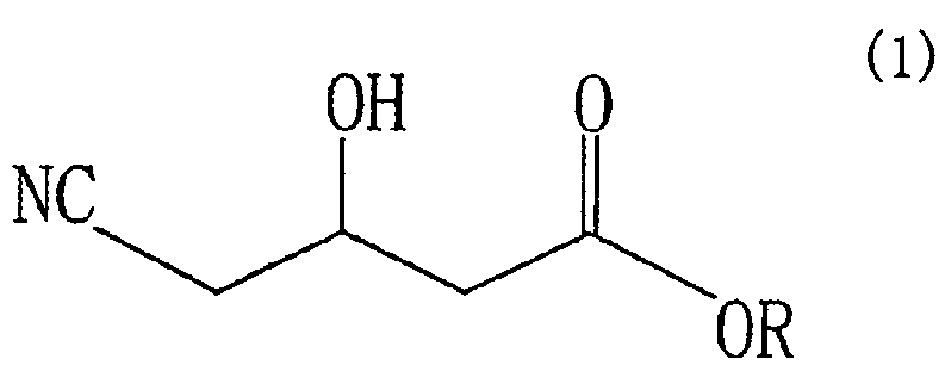
A process for preparing (R)-4-cyano-3-hydroxybutyric acid ester
A kind of hydroxybutyrate, the preparation process technology, applied in the field of optically pure 4-cyano-3-hydroxybutyrate products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] The following examples are intended to illustrate the present invention and should not be considered as defining the scope of the present invention due to additional requirements. Example 1: Preparation of (S)-3-acetoxy-4-bromobutanoic acid
[0022] Add (S)-3-hydroxybutyrolactone (102 grams) and 30% hydrobromic acid (675 grams, 2.5 equivalents) to a two-liter three-necked flask equipped with a reflux condenser, a thermometer and a mechanical stirrer. . The mixture was stirred at 40°C for 3 hours. After cooling the reaction mixture, further dichloromethane (1000 mL) was added to the mixture. The reaction mixture was rinsed with aqueous sodium acetate. The organic layer was separated, dried over magnesium sulfate and concentrated to yield (S)-3-acetoxy-4-bromobutanoic acid (213 g, 95%).
example 2
[0023] 1H-NMR (D 2 O, ppm): δ21(S, 3H, CH 3 COO), 2.8-2.9 (dd, 2H, CH 2 COOH), 3.5-3.6 (dd, 2H, BrCH 2 CH), 5.3-5.4 (m, 1H, BrCH 2 CH) Example 2: Preparation of (S)-3,4-epoxybutyric acid sodium salt
[0024] A 5 liter flask was charged with (S)-3-acetoxy-4-bromobutanoic acid from Example 1. Slowly add 1NC normality sodium hydroxide aqueous solution and keep the temperature below zero. The reaction mixture was stirred at 0°C for 1 hour. The relatively pure sodium salt of (S)-3,4-epoxybutyric acid can be detected by NMR.
[0025] 1H-NMR (D 2 O, ppm): δ2.3-2.5 (m, 2H, CH 2 -CO 2 Na), 2.6-2.9(m, 2H), 3.2-3.3(m, 1H)
[0026] 13C-NMR (D 2 O, ppm): δ40.87 (-CH 2 -CO 2 Na), 48.24 (4-CH 2 ), 51.08 (3-CH), 179.41 (-CO 2 Na) Example 3: Preparation of (S)-3,4-epoxybutyric acid potassium salt
[0027] A 5 liter flask was charged with (S)-3-acetoxy-4-bromobutanoic acid from Example 1. Aqueous 1 N (normal strength) potassium hydroxide (3 L, 3 mol) was slowly added while main...
example 4
[0029] 13C-NMR (D 2 O): δ40.87(-CH 2 -CO 2 K), 48.24 (4-CH 2 ), 51.08 (3-CH), 179.41 (-CO 2 K) Example 4: Preparation of (S)-3,4-epoxybutyric acid calcium salt
[0030] A 2-liter three-neck flask equipped with a thermometer, a pH meter and a mechanical stirrer was charged with distilled water (1 liter), (S)-3-acetoxy-4-bromobutanoic acid (90 g, 0.4 mol) and Calcium hydroxide (45 g, 0.6 mol). The reaction mixture was stirred at 0-5°C for 2 hours to yield (S)-3-4-epoxybutyric acid calcium salt. More than 99% conversion was determined by NMR.
[0031] 1H-NMR (D 2 O, ppm): δ2.3-2.5 (m, 2H, CH 2 -CO 2 Ca), 2.5-2.8(m, 2H, 4-H), 3.2-3.3(m, 1H, 3-H) Example 5: Preparation of (S)-3,4-epoxybutanoic acid tetrabutylammonium salt
[0032] A 2-liter three-neck flask equipped with a thermometer, a pH meter and a mechanical stirrer was charged with distilled water (1 liter), (S)-3-acetoxy-4-bromobutanoic acid (90 g, 0.4 mol) and To methanol (1.2 L, 1.2 mol) was added 1.0 M tetrabut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com