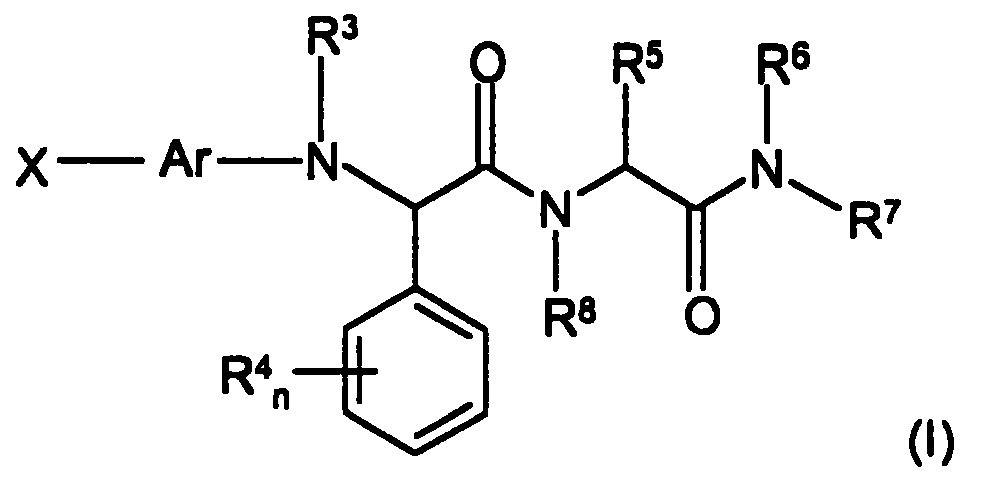
Novel compounds inhibiting factor Xa activity
A compound and composition technology, applied in the field of new compounds that inhibit the activity of factor Xa, can solve the problems such as the need to monitor patients, the scope of action of Xa inhibitors and the scope of side effects are not fully investigated, and the dose needs to be adjusted, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] 0.05M of 2-(3,4,5-trihydroxy-6-hydroxymethyl-tetrahydropyran-2-yloxy)-benzaldehyde in methanol (helicin), 0.05M of 3-amino A solution of benzamidine dihydrochloride in methanol and 0.05 M of 2-isocyano-1-[4-(2-methoxy-phenyl)-piperazin-1-yl]-ethanone in methanol The solution in was reacted at room temperature for 24 hours in a closed vessel. After evaporation of the solvent, the product was subjected to liquid chromatography and mass spectrometry to determine the structure of the final product. The product 2-(3-amidino-phenylamino)-N-{2-[4-(2-methoxy) was purified by liquid chromatography on a reverse phase column using a water / acetonitrile gradient as eluent -Phenyl)-piperazin-1-yl]-2-oxo-ethyl}-2-[2-(3,4,5-trihydroxy-6-hydroxymethyltetrahydropyran-2-yl Oxy)-phenyl]-acetamide.
[0095] C 34 h 42 N 6 o 9 (678.7486)
[0096] ESI-TOF-MS: 679[M+H] +
Embodiment 2
[0098] Analogously to Example 1, and using corresponding suitable starting materials, 2-(3-amidino-phenylamino)-N-{2-[4-(4-methoxy-phenyl)-piperazine is obtained -1-yl]-2-oxo-ethyl}-2-[2-(3,4,5-trihydroxy-6-hydroxymethyltetrahydropyran-2-yloxy)-phenyl] - Acetamide.
[0099] C 34 h 42 N 6 o 9 (678.7486)
[0100] ESI-TOF-MS: 679[M+H] +
Embodiment 3
[0102] Similar to Example 1, and using corresponding suitable starting materials, 2-biphenyl-4-yl 2-(3-amidino-phenylamino)-N-{2-[4-(2-methyl Oxy-phenyl)-piperazin-1-yl]-2-oxo-ethyl}-acetamide.
[0103] C 34 h 36 N 6 o 3 (576.7044)
[0104] ESI-TOF-MS: 577[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com