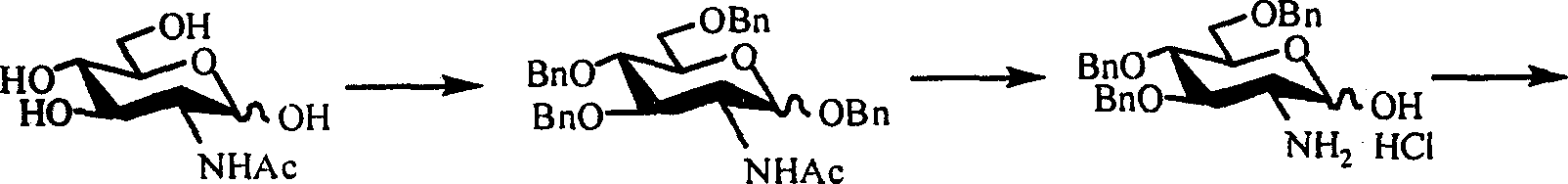Acetamido glucose devicative of indomethacin and its synthetic method and use
A technology of glucose derivatives and acetylamino, which is applied in the direction of sugar derivatives, drug combinations, medical preparations containing active ingredients, etc., and can solve the problem of inhibiting etiology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
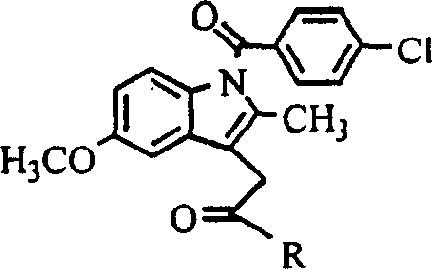
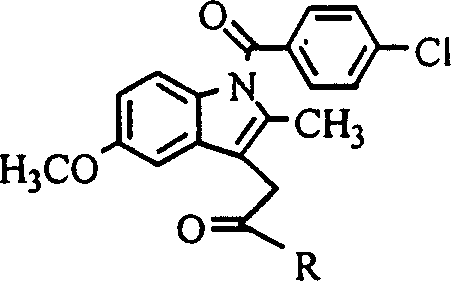
[0010] Example 1: 1-O-[1-(4-chloro-benzoyl)-5-methoxy-2-methyl-3-indole-acetoxy]-2-acetylamino-2-deoxy - Synthesis of α-D-glucose (hereinafter referred to as compound 1)
[0011] Step 1: Benzyl protection
[0012]
[0013] Compound 3 Compound 4 Compound 5
[0014]
[0015] Compound 6 Compound 7
[0016] Dissolve 1mmol of acetylglucosamine (hereinafter referred to as compound 3) in 10mL of N,N-dimethylformamide (hereinafter referred to as DMF), react in ice bath for 30min, add 6mmol of benzyl bromide, stir for 10min, then add 9.3mmol of barium oxide , 1.7 mmol of barium hydroxide octahydrate and a catalytic amount of tetrabutylammonium iodide. The reaction was carried out in an ice bath for 5 h and at room temperature overnight. Add 15 mL of dichloromethane to dilute, filter with diatomaceous earth to remove insoluble matter, and wash the filter cake several times with dichloromethane. The filtrate was evaporated to dryness, dissolved in dichloromethane...
Embodiment 2
[0028] Example 2: 6-O-[1-(4-chloro-benzoyl)-5-methoxy-2-methyl-3-indole-acetoxy]-2-acetylamino-2-deoxy - Synthesis of α-D-glucose (hereinafter referred to as compound 2)
[0029] Step 1: Benzyl protection
[0030]
[0031] Compound 3 Compound 9
[0032] Stir the mixture of 0.01 mol of compound 3 and 20 mL of benzyl alcohol evenly, then add 0.1 mL of boron trifluoride ether, and heat to reflux. After the reaction was detected by TLC, the reaction solution was cooled to 0° C., and 120 mL of ether was slowly added, and a large amount of precipitates precipitated out. After standing overnight at 4°C, filter, wash the filter cake with ether, and dry to obtain 2.60 g of white solid 1-O-benzyl-2-acetylamino-2-deoxy-D-glucose (hereinafter referred to as compound 9). Rate 83.6%, R f 0.30 (chloroform:methanol=6:1). When the feeding amount of compound 3 is 0.01mol, the feeding amount of the benzyl alcohol is 5-50ml, and the feeding amount of the boron trifluoride ether is 0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com