Compounds of podophyllotoxins, application and preparation method
A compound and alcohol ester technology, applied in the field of new podophyllin compounds, can solve the problems of strong drug resistance, poor water solubility, poor oral effect, etc. Good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
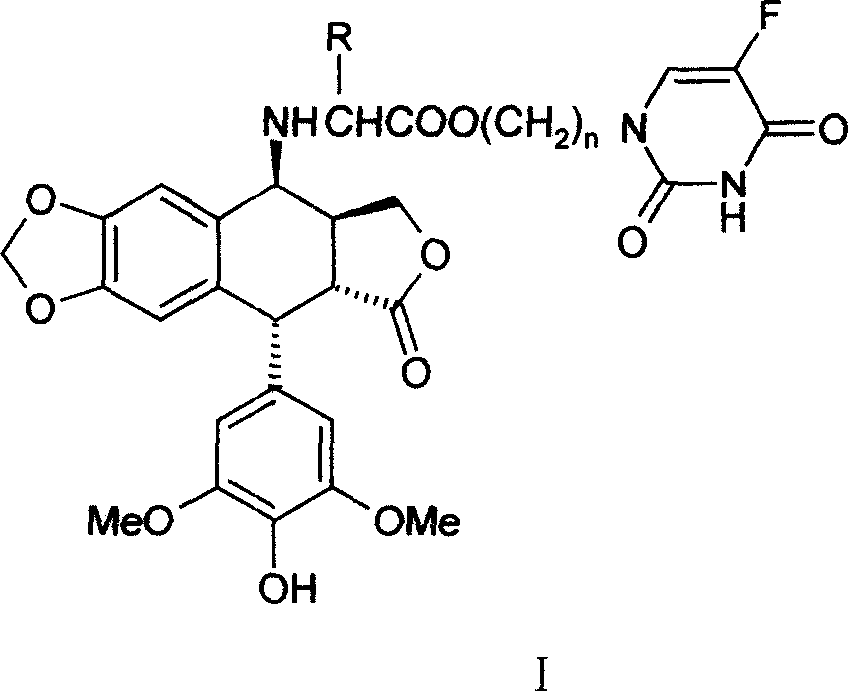
[0022] 4′-norepipodophyllotoxin of 4β-4-deoxy-nitrogen substituted valine (5-Fu yl)propanol ester
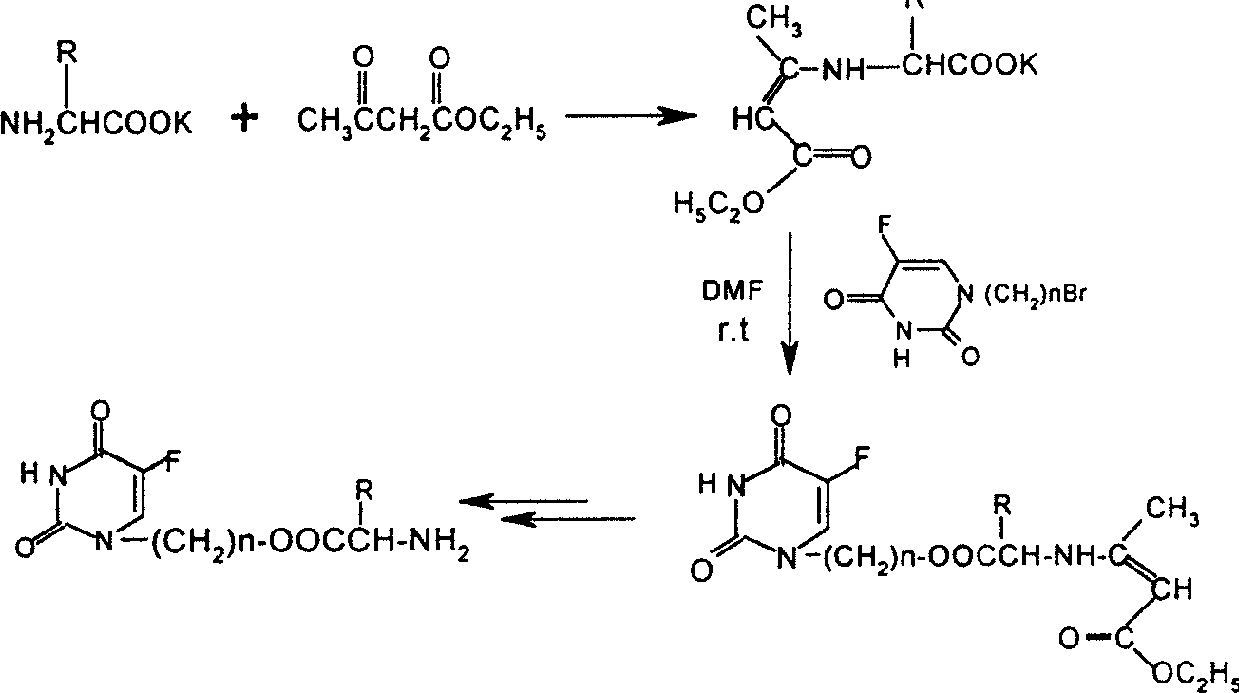
[0023] Take 10mmol ethyl acetoacetate protected valamide potassium salt and an equivalent amount of N 1 -Bromopropyl 5-Fu was dissolved in dry DMF, and a trace amount of KI was added to react at room temperature for 24 hours. KBr was filtered off, the filtrate was distilled off DMF under reduced pressure, dissolved in 70ml of ethyl acetate, washed with 50ml of 1M sodium bicarbonate and 2×50ml of water, and dried overnight with anhydrous sodium sulfate. Evaporate the solution under reduced pressure, add 20ml of 1M methanol solution of hydrogen chloride and stir at room temperature for 2 hours, evaporate the solvent under reduced pressure, dissolve with 15ml of water, and extract with 2×15ml of ethyl acetate, distill the water layer under reduced pressure to obtain the N 1 - The hydrochloride salt of 5-Fuyl-propanol ester. The above hydrochloride was added to 20ml of dichloromet...
Embodiment 2
[0029] 4′-norepipodophyllotoxin of 4β-4-deoxy-nitrogen substituted 5-Fu propyl ester of phenylalanine
[0030] The experimental procedure is the same as in Example 1, except that valine is replaced by phenylalanine. The detection data of the product obtained by the reaction are as follows:
[0031] Yield: 33%; m.p.113-115°C; 1 HNMR (CDCl 3 , TMS, 300MHz): 9.34(br, 1H, CONH*CO), 7.32~7.09(m, 5H, Ar-H), 7.03(d, 1H, H-6(5-Fu), J=5.1Hz) , 6.45(s, 1H, H-5), 6.33(s, 1H, H-8), 6.22(s, 2H, H-2', 6'), 5.95(d, 2H, OCH 2 O, J = 1.8Hz), 5.46 (br, 1H, OH), 4.45 (d, 1H, H-1, J = 5.4Hz), 4.26 (d, 2H, H-11, J = 8.7Hz), 4.10 (t, 2H, OCH 2 , J=6.0Hz), 4.02(d, 1H, H-4, J=3.9Hz), 3.74(s, 6H, OCH 3 ), 3.50(t, 2H, CH 2 -5Fu, J=6.6Hz), 3.38(t, CH*CH 2 Ar, J = 6.9Hz) 3.16 (dd, 1H, H-2, J 1 =13.5, 5.4Hz), 3.01 (m, 2H, ArCH 2 -), 2.75(m, 1H, H-3), 1.85(m, 2H, -CH 2 -);IR(KBr)υcm -1 , 3440, 2918, 1772, 1697, 1481, 1230, 1112; HRMS (ESI) C 37 h 36 o 11 N 3 F, theoretical value (M+Na), 740...
Embodiment 3
[0034] 4′-norepipodophyllotoxin of 4β-4-deoxy-nitrogen substituted isoleucine 5-Fu propyl ester
[0035] The experimental procedure is the same as in Example 1, except that the valine in Example 1 is replaced with isoleucine. The detection data of the product obtained by the reaction are as follows:
[0036] Yield: 38%; m.p.118-120°C; 1 HNMR (CDCl 3 , TMS, 300MHz): 8.84 (br, 1H, CONH*CO), 7.28 (d, 1H, H-6(5-Fu), J=5.4Hz), 6.65 (s, 1H, H-5), 6.50 (s, 1H, H-8), 6.24 (s, 2H, H-2', 6'), 5.97 (d, 2H, OCH 2 O, J = 5.4Hz), 5.41 (br, 1H, OH), 4.55 (d, 1H, H-1, J = 5.7Hz), 4.33 (s, 1H, OCHa), 4.30 (s, 1H, OCHb) , 4.23(m, 1H, H-4), 3.95(d, 1H, H-11a), 3.82(t, 2H, CH 2 -5Fu, J=6.0Hz), 3.76(s, 6H, OCH 3 ), 3.60(m, 1H, H-11b), 3.47(s, 1H, OH), 3.42(dd, 1H, H-2, J=13.8, 5.4Hz), 3.29(d, 1H, CH*COO, J=5.4Hz), 2.85(m, 1H, H-3), 2.32(m, 1H, CHMe 2 ), 2.08 (m, 2H, -CH 2 -), 1.74(m, 1H, EtCH*Me), 1.41(m, 1H, MeCHa*CHMe), 1.19(m, 1H, MeCHb*CHMe) 0.89(m, 6H, 2CH 3 ); IR(KBr)υcm -1 , 3439...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com