Taxone bromated analog with anticancer activity and preparation method thereof
An anticancer activity, taxane technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
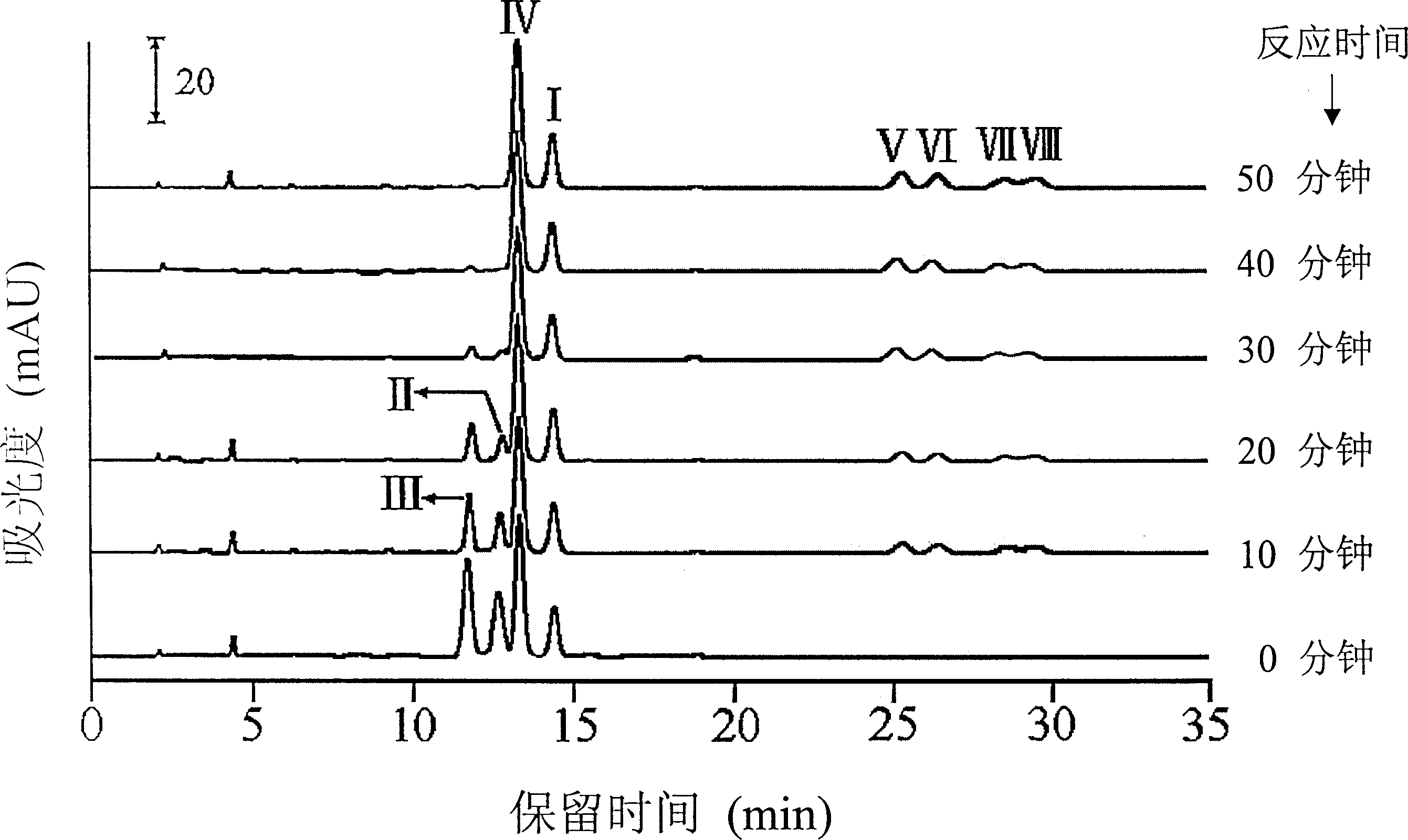
[0063] Bromination of partially purified mixtures containing 7-epi-10-deacetyl-cephalomannine, cephalomannine and other taxanes
[0064] 3 g of the crude extract of Taxus chinensis was dissolved in 30 ml of chloroform under the action of a magnetic stirrer, and cooled in an ice-water bath for 0.50 hour. Slowly add 100 μl of liquid bromine dropwise to the three reaction vessels respectively, and react in an ice-water bath at 0° C., protected from light, and stirred. From the dropwise addition of liquid bromine reaction, every 10min, quantitatively pipette 1ml of the reaction solution from the reaction vessel into the separatory funnel, and then add 5ml of 10% Na 2 S 2 o 3 The solution reduces excess bromine therein. Use a dry 5ml small test tube to collect the lower organic phase, pipette 0.50ml of the solution from the separated organic phase into a dry eggplant-shaped bottle, evaporate to dryness at room temperature, dissolve in 2.50ml of methanol, and detect with HPLC . ...
Embodiment 2
[0066] Normal Phase Preparative Chromatographic Separation of Bromination Reaction Products
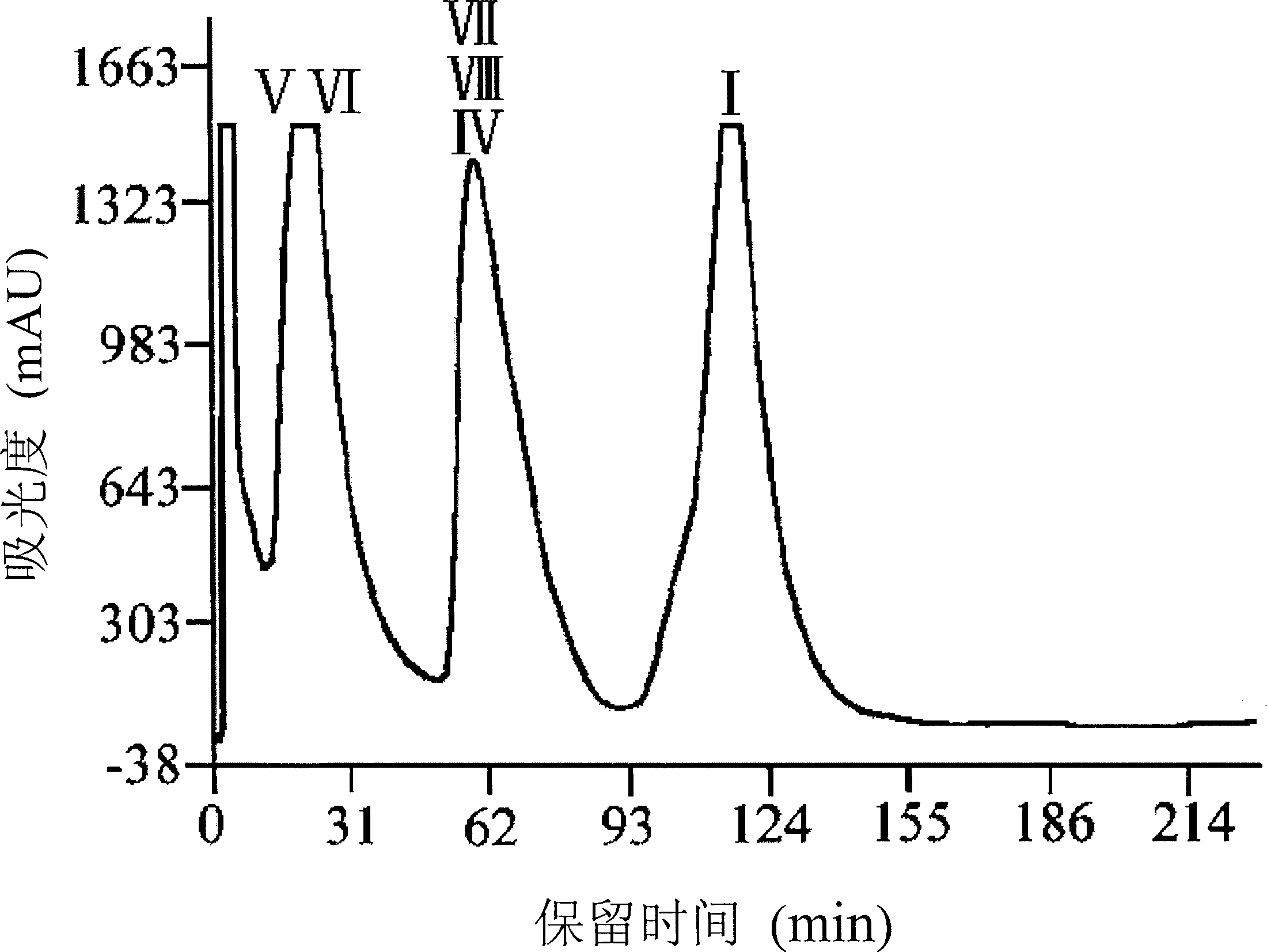
[0067] Dissolve 3 g of the above reaction mixture in 50 ml of 1:1 ethyl acetate and n-hexane, use a P280 high-pressure constant-flow pump to inject the sample, and use ethyl acetate:n-hexane (1:1~1:1.5) as the flow equal degree elution , A total of 30 bottles of fractions were collected, 100ml / bottle. The fractions were evaporated to dryness under reduced pressure and detected by reverse phase HPLC. All the fractions were divided into 3 parts and merged respectively, wherein fractions 1 to 10 were mainly two diastereoisomers of 2", 3"-dibromo-7-table-10-deacetyl-cephalomannine, Fractions 11-22 are mainly two diastereoisomers of 7-table 10-deacetyl paclitaxel and 2”, 3”-dibromocephalomannine, and fractions 23-30 are high-purity paclitaxel. The individual fractions were evaporated to dryness under reduced pressure. The chromatogram of the normal phase preparation process and the resu...
Embodiment 3
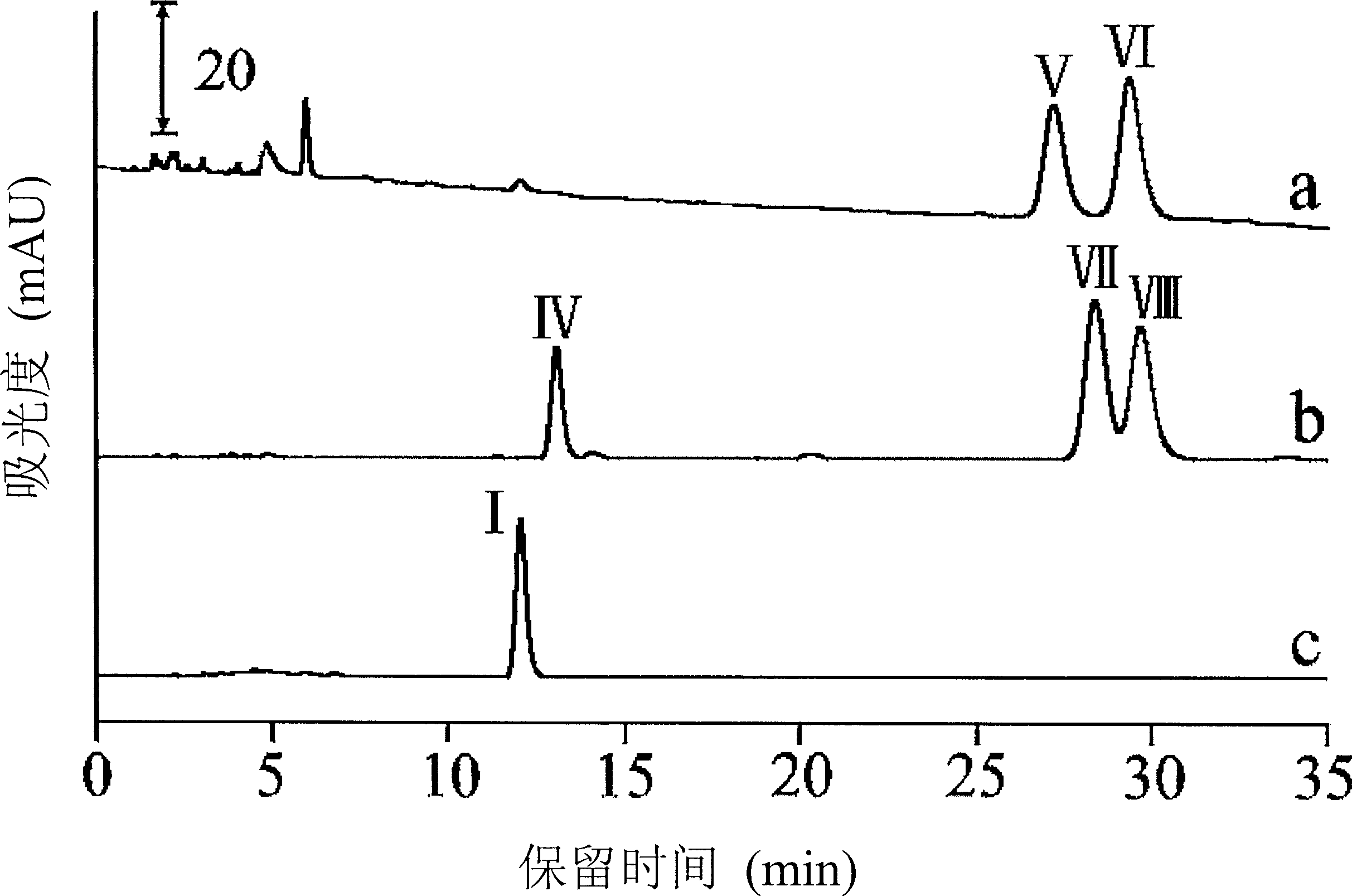
[0069] Separation of individual diastereoisomers of 2", 3"-dibromo-7-epi-10-deacetyl-cephalomannine and 2", 3"-dibromocephalomannine
[0070] The separation of the individual diastereoisomers of 2", 3"-dibromo-7-epi-10-deacetyl-cephalomannine and 2", 3"-dibromocephalomannine was by reversed-phase semi-preparative high-performance liquid chromatography system. 100 mg of dried solid mainly containing two diastereoisomers of 2", 3"-dibromo-7-epi-10-deacetyl-cephalomannine were dissolved in 1.5 ml of methanol, and then The sample was washed with water: acetonitrile (60:40) mobile phase, and the two diastereoisomers elute at 27.3 min and 29.1 min, respectively. The separation process of the diastereoisomers of single 2", 3"-dibromocephalomannine is basically the same as the above-mentioned process. After the injection, the mobile phase of water: acetonitrile (62:38) is used for elution, and the two Two diastereomers elute at 28.4 min and 29.6 min. The four individual diastereome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com