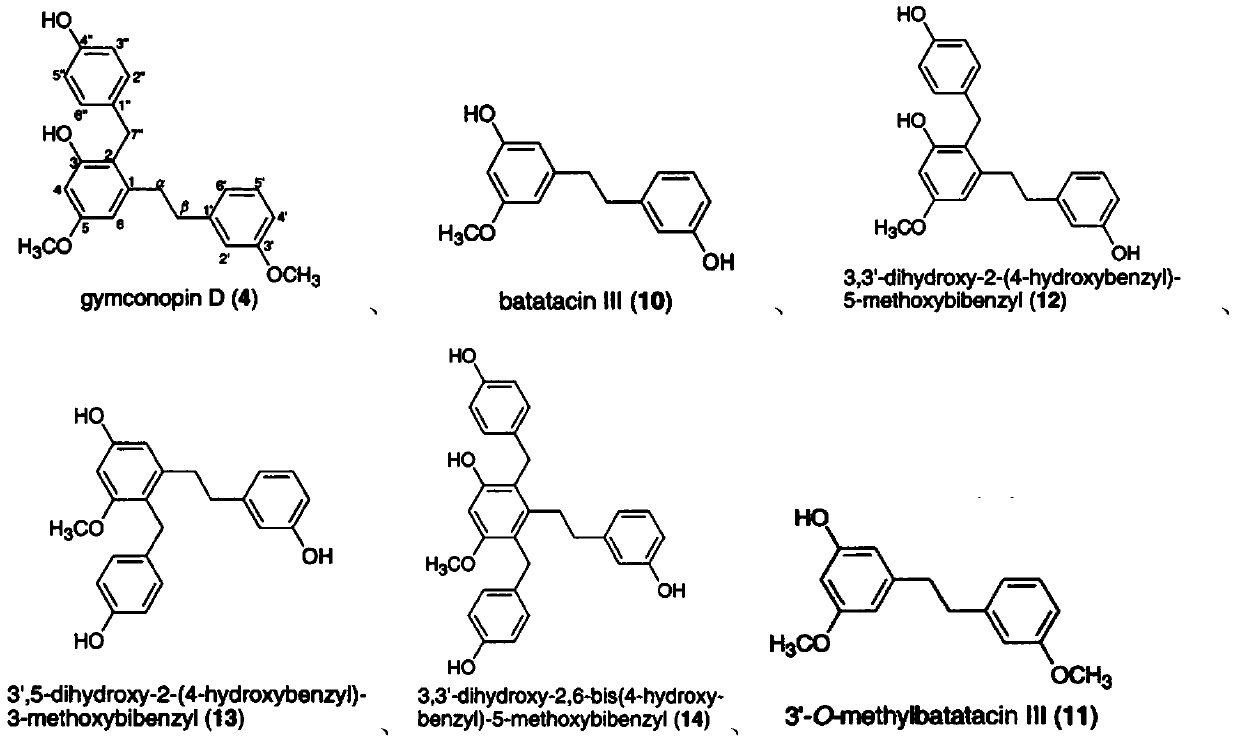
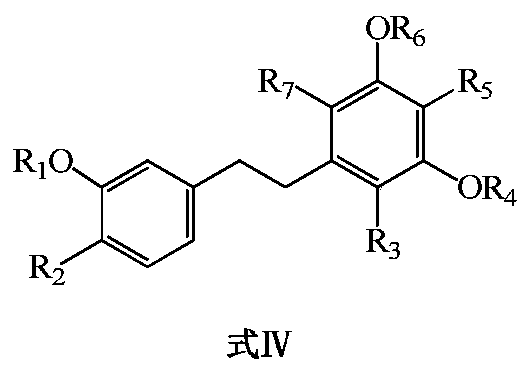
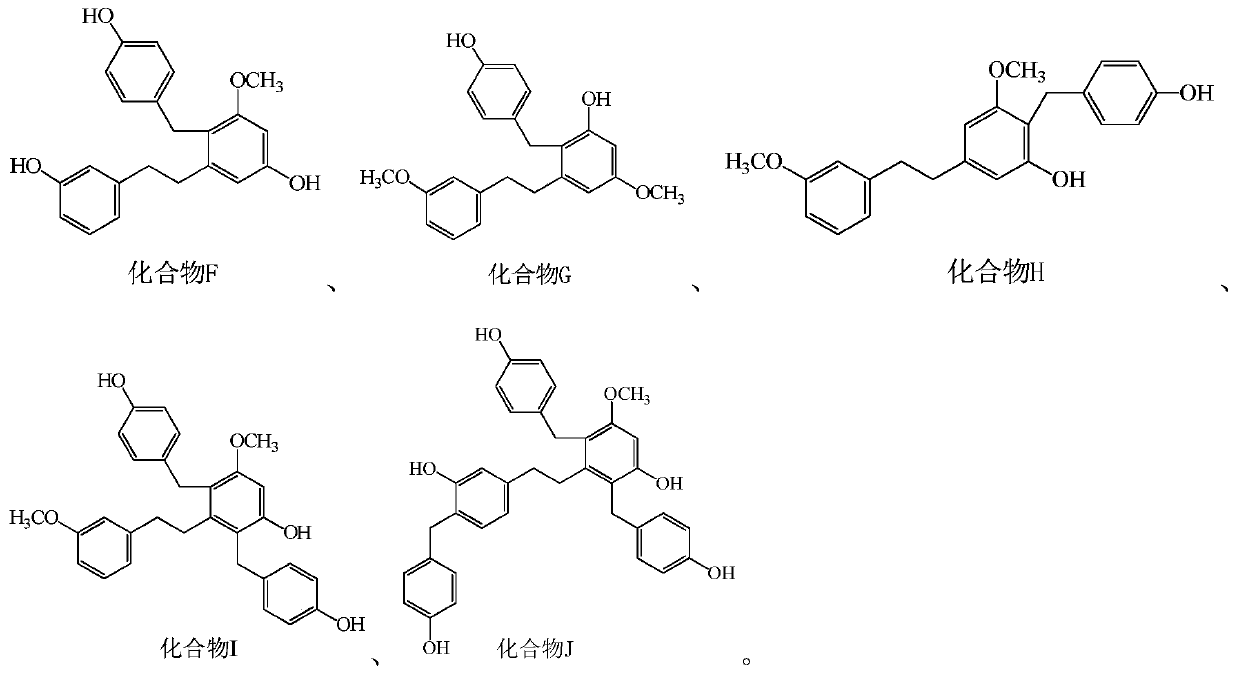
Bibenzyl compound and its preparation method and application in the preparation of antitumor drugs
A compound and application technology, applied in the field of preparation of anti-tumor drugs, can solve the problems that there are no research reports on anti-tumor drugs of bibenzyl compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1, the extraction and structural identification of compound F
[0117] (1) Extraction of medicinal materials:
[0118] Dried white and medicinal material coarse powder (3kg), reflux extraction with 95% ethanol 3 times (30L×3), 2h each time;
[0119] (2) Separation and purification of components:
[0120] 1. Ethanol extract (90L) was concentrated and dried under reduced pressure to obtain 510g of semi-solid liquid extract;
[0121] ②. Disperse the semi-solid liquid extract (500g) with water (5L), extract with ethyl acetate and n-butanol (20L) successively, combine the ethyl acetate part, recover the solvent under reduced pressure, and obtain 160g of ethyl acetate extract;
[0122] 3. Adopt silica gel column chromatography to separate ethyl acetate extract (160g), petroleum ether: acetone carries out gradient elution, and the eluent is detected by thin-layer chromatography, and obtains the elution when petroleum ether: acetone=30:1 Liquid Fr.16;
[0123] The c...
Embodiment 2
[0137] Example 2, the extraction and structural identification of compound G
[0138] (1) Extraction of medicinal materials:
[0139] Dried white and medicinal material coarse powder (3kg), reflux extraction with 95% ethanol 3 times (30L×3), 2h each time;
[0140] (2) Separation and purification of components:
[0141] 1. Ethanol extract (90L) was concentrated and dried under reduced pressure to obtain 510g of semi-solid liquid extract;
[0142] ②. Disperse the semi-solid liquid extract (500g) with water (5L), extract with ethyl acetate and n-butanol (20L) successively, combine the ethyl acetate part, recover the solvent under reduced pressure, and obtain 160g of ethyl acetate extract;
[0143] 3. Adopt silica gel column chromatography to separate ethyl acetate extract (160g), petroleum ether: acetone carries out gradient elution, and the eluent is detected by thin-layer chromatography, and obtains the elution when petroleum ether: acetone=30:1 Liquid Fr.16;
[0144] The c...
Embodiment 3
[0157] Example 3, the extraction and structural identification of compound H
[0158] (1) Extraction of medicinal materials:
[0159] Dried white and medicinal material coarse powder (3kg), reflux extraction with 95% ethanol 3 times (30L×3), 2h each time;
[0160](2) Separation and purification of components:
[0161] 1. Ethanol extract (90L) was concentrated and dried under reduced pressure to obtain 510g of semi-solid liquid extract;
[0162] ②. Disperse the semi-solid liquid extract (500g) with water (5L), extract with ethyl acetate and n-butanol (20L) successively, combine the ethyl acetate part, recover the solvent under reduced pressure, and obtain 160g of ethyl acetate extract;
[0163] 3. Adopt silica gel column chromatography to separate ethyl acetate extract (160g), petroleum ether: acetone carries out gradient elution, and the eluent is detected by thin-layer chromatography, and obtains the elution when petroleum ether: acetone=30:1 Liquid Fr.16;
[0164] The co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com