Resin compound composition as well as preparation method and application thereof
A resin compound and composition technology, which is applied in the directions of drug combination, active ingredient of heterocyclic compound, pharmaceutical formula, etc., can solve the problems such as taste masking of undisclosed dextromethorphan quinidine composition, and achieves simple preparation process and improved Medication experience, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] It is called 1.6g of hydrochide, right Mishafin and 1.6g of sulfinin, and add 400ml purified water to dissolve. Under 37 ° C, add 1g ion exchange resin, stir for 20 hours, centrifugal, obtained the dry body and was washed and dried to obtain hydroceroideylumin resin complexes and sulfinin dine resin complexes.
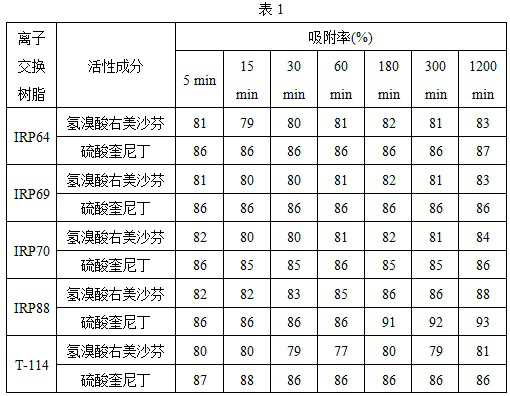
[0028] In the process of preparing the resin complex, take 2ml solution at different time points (min), centrifugation, detect the concentration of the remaining active ingredients in the solution after filtering, calculate the adsorption rate of ion exchange resin to the right Michafin or Quininin ( %), The result is shown in Table 1.
[0029]
[0030] The results showed that the adsorption of various ion exchanges resin has a fast adsorption of hydromodoline or sulfininininininin or sulfinin, and the adsorption rate can reach more than 80%at 5min.
[0031]The right Michafin resin complexes and Quinine resin complexes artificial taste. The method of taste taste i...
Embodiment 2
[0038] It is said that the hydromodiacomy and sulfinininin dite dines are prepared, and the aqueous solution of 2.33 mg / ml is prepared. After adding IRP88 resin under 37 ° C, stir, centrifugation, and solid washing are dry. Resin complex.
[0039] According to the above methods, the right Michafin resin complexes and Quinine resin complexes with different quality ratios. The artificial taste method in the embodiment 1 is used to check the smell effect of the resin complexion made by the resin composite, and the results are shown in Table 4.
[0040]
[0041] The results show that with the increase of IRP88, the effects of IRP88 on Right Michafin and Quinine are enhanced. When the quality of IRP88 is two times or more of the quality of the hydromodium hydromodolic acid or sulfininin, it has a good effect on the mask and Quinindin. However, in combination with embodiments 1, 1G IPR88 has a masculinity result of 1.6g of hydrochride and sulfinin, which indicates that the concentrati...
Embodiment 3
[0043] Called 1.2g of hydrochride, right Mishafin and 1.2g of sulfinin, add an appropriate amount of exfoliating water to prepare the concentration of hydroceroladium hydrocero with a concentration of 1 mg / ml, 2mg / ml, 3mg / ml, and 4 mg / ml. Sour Masifen solution and sulfininin solution. Under 37 ° C, invested 2.4g of IRP88 resin, respectively. After centrifugal and solid, the right Michafin resin complex or Quinine resin complexes were obtained.
[0044] Calculate the adsorption rate (%) of IRP88 according to the same method of Example 1, and the results are shown in Table 5.
[0045]
[0046] The results show that when the amount of resin additions is twice the quality of the right Michafin or Quinine, the adsorption rate of IRP88 resin on Right Michafin or Quinitin is increased with the concentration of Right Michafin or Quinine. When the concentration of the solution of the deeds of hydride or sulfininine is 2 mg / ml or larger, the adsorption rate of IRP88 resin to Youmeishain o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com