Synthesis method of 2-hydroxy-2-methylsuccinic acid without metal participation
A technique for the synthesis of methylsuccinic acid and its synthesis method, which is applied in the field of synthesis of 2-hydroxy-2-methylsuccinic acid, and can solve the problem of low efficiency, high cost and 2-hydroxy-2-methylsuccinic acid production process Complicated problems, to achieve the effect of simple process equipment requirements, mild operating conditions, and large industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
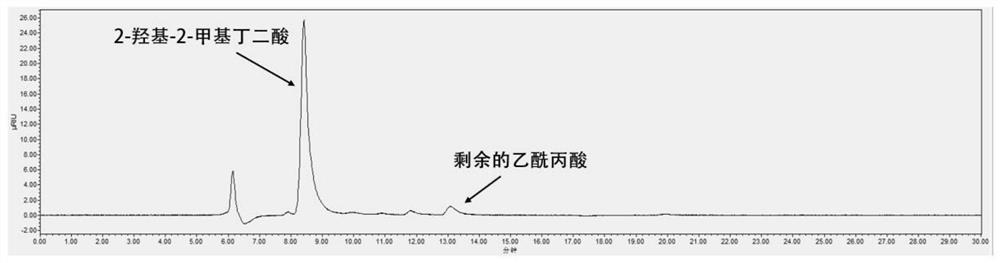
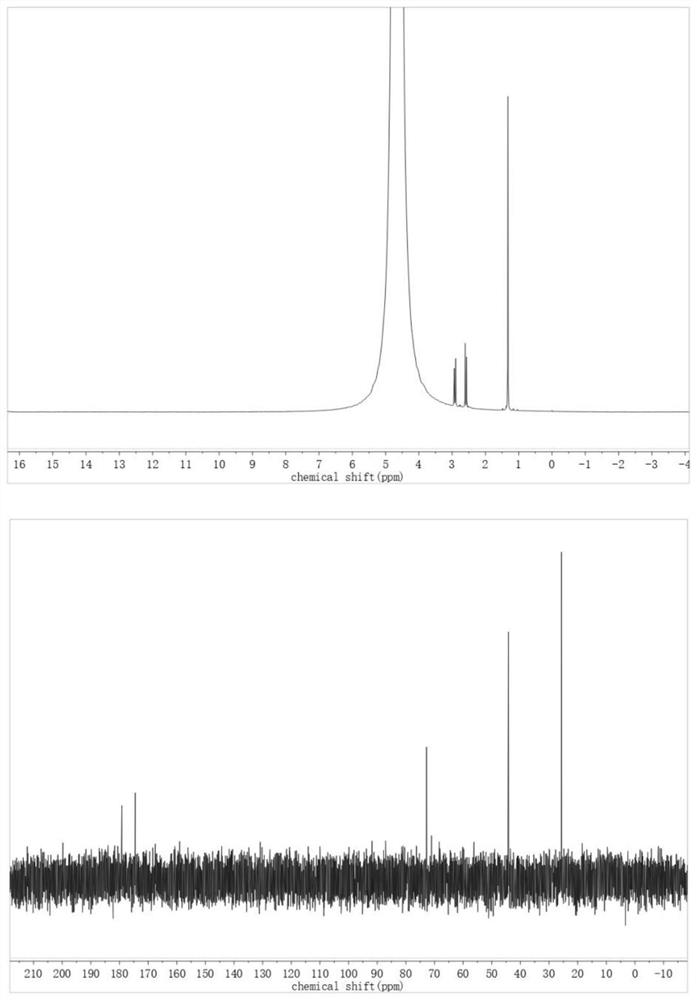
[0020] Add 0.15mmol levulinic acid, 50mg CaO, 50mg activated carbon in sequence to a 10mL high temperature and high pressure reaction kettle, seal the reaction kettle, fill with oxygen (0.5Mpa), stir and heat to 90°C for 0.5h. The reaction kettle was cooled to room temperature in an ice-water bath, and the aqueous phase was collected and detected. The conversion rate of levulinic acid was 96%, and the yield of 2-hydroxy-2-methylsuccinic acid was 89%. The product was detected by H NMR and C NMR. confirmed.
[0021] The levulinic acid conversion rate, the detection and calculation of the 2-hydroxy-2-methylsuccinic acid yield are carried out according to the following methods:
[0022] The detection instrument for the concentration of levulinic acid and 2-hydroxy-2-methylsuccinic acid is a liquid chromatograph Waters AcquityHclass (Coregel 107 chromatographic column: 7.8mm*300mm).
[0023] Calculation of levulinic acid conversion:
[0024] Conversion rate of levulinic acid=(1-l...
Embodiment 2
[0028] Add 0.75mmol levulinic acid, 250mg CaO, 250mg activated carbon powder in sequence to a 50mL high temperature and high pressure reaction kettle, seal the reaction kettle, fill with oxygen (0.5Mpa), stir and heat to 90°C for 0.5h. The reaction kettle was cooled to room temperature in an ice-water bath, and the aqueous phase was collected and detected. The conversion rate of levulinic acid was 95%, and the yield of 2-hydroxy-2-methylsuccinic acid was 81%. The product was detected by H NMR and C NMR. confirmed.
Embodiment 3
[0030] Add 0.15mmol levulinic acid, 50mg CaO, 50mg activated carbon calcined at 900°C for 4h in nitrogen atmosphere into a 10mL high temperature and high pressure reaction kettle in turn, seal the reaction kettle, fill with oxygen (0.5Mpa), stir and heat to 90°C for 0.5h . The reaction kettle was cooled to room temperature in an ice-water bath, and the aqueous phase was collected and detected. The conversion rate of levulinic acid was 86%, and the yield of 2-hydroxy-2-methylsuccinic acid was 56%. The product was detected by H NMR and C NMR. confirmed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com