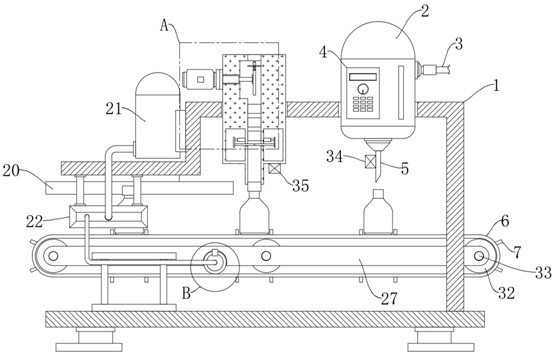
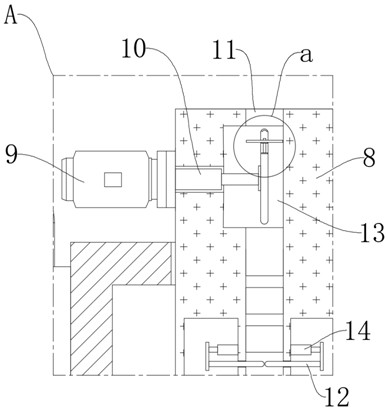
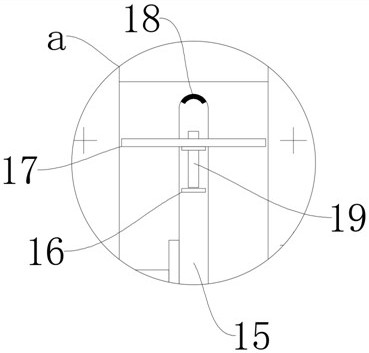
Oral solution containing calcitriol and preparation method thereof
A technology of calcitriol and oral solution, which is applied in medical preparations containing active ingredients, medical preparations without active ingredients, and liquid treatment, etc., can solve the problem that calcitriol oral solution is not imported into China, and achieve The effect of a high degree of automation and a faster replenishment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] An oral solution containing calcitriol, comprising the following components by weight:
[0077] Calcitriol: 0.290 parts;
[0078] Butyl hydroxyanisole: 29.7 parts;
[0079] Dibutylhydroxytoluene: 29.8 parts;
[0080] Medium-chain triglycerides: 299,987 parts.
[0081] A preparation method of the above-mentioned oral solution containing calcitriol, comprising the steps:
[0082] S100, weighing: turn on the yellow light in the weighing room, weigh calcitriol, medium-chain triglycerides, butylated hydroxyanisole, and dibutylated hydroxytoluene according to parts by weight, and double-check;
[0083] S200, preparation: wherein, the preparation of solution one:
[0084] Turn on the vacuum pump to evacuate, make the pressure in the dosing tank reach -0.06 ~ MPa, close the vacuum pump; add 85% of the total feeding amount of medium chain triglycerides into the dosing tank through vacuum feeding;
[0085] Open the breathing valve to restore the air pressure in the tank to a...
Embodiment 2
[0100] An oral solution containing calcitriol, comprising the following components by weight:
[0101] Calcitriol: 0.305 parts;
[0102] Butyl hydroxyanisole: 30.3 parts;
[0103] Dibutylhydroxytoluene: 30.2 parts;
[0104] Medium-chain triglycerides: 300,012 servings.
[0105] A preparation method of the above-mentioned oral solution containing calcitriol, comprising the steps:
[0106] S100, weighing: turn on the yellow light in the weighing room, weigh calcitriol, medium-chain triglycerides, butylated hydroxyanisole, and dibutylated hydroxytoluene according to parts by weight, and double-check;
[0107] S200, preparation: wherein, the preparation of solution one:
[0108] Turn on the vacuum pump to evacuate, make the pressure in the dosing tank reach -0.1MPa, and close the vacuum pump; add 85% of the medium chain triglycerides of the total feeding amount into the dosing tank through vacuum feeding;
[0109] Open the breathing valve to restore the air pressure in the ta...
Embodiment 3
[0124] An oral solution containing calcitriol, comprising the following components by weight:
[0125] Calcitriol: 0.300 parts;
[0126] Butyl hydroxyanisole: 30.0 parts;
[0127] Dibutylhydroxytoluene: 30.0 parts;
[0128] Medium chain triglycerides: 2,300,000 servings.
[0129] A preparation method of the above-mentioned oral solution containing calcitriol, comprising the steps:
[0130] S100, weighing: turn on the yellow light in the weighing room, weigh calcitriol, medium-chain triglycerides, butylated hydroxyanisole, and dibutylated hydroxytoluene according to parts by weight, and double-check;
[0131] S200, preparation: wherein, the preparation of solution one:
[0132] Turn on the vacuum pump to evacuate, make the pressure in the dosing tank reach -0.08MPa, and close the vacuum pump; add 85% of the medium chain triglycerides of the total feeding amount into the dosing tank through vacuum feeding;
[0133] Open the breathing valve to restore the air pressure in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com