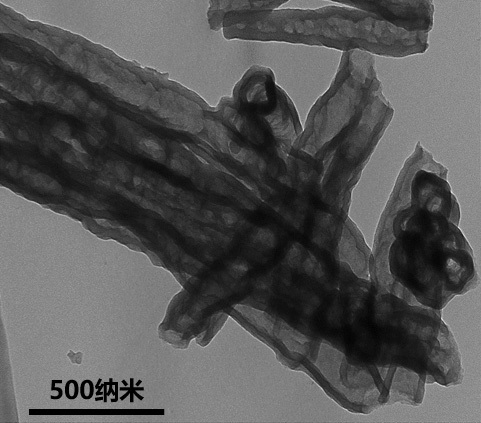
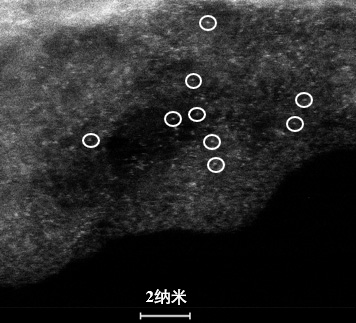
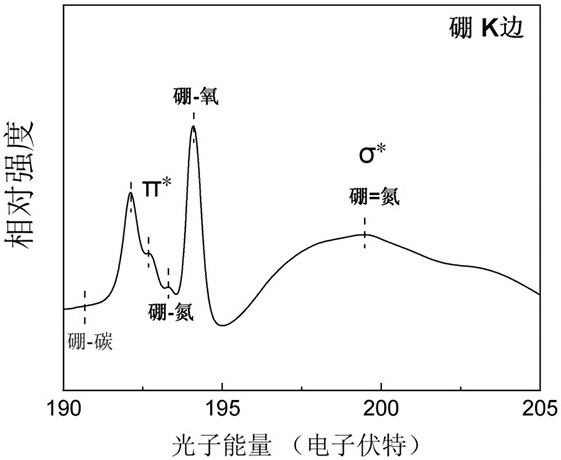
Preparation method and application of boron-nitrogen co-coordinated copper monatomic catalyst
A catalyst and atomic technology, which is applied in the field of preparation of boron-nitrogen co-coordinated copper single-atom catalysts, can solve the problems of difficult stable conversion, limited composition, loss of high selectivity of single-atom centers, etc., and achieves easy separation and less special equipment , the effect of step simplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of BCN-Cu single-atom catalyst
[0034] (1) Add 20.02g of urea, 2.01g of PEG-2000, 599mg of boric acid, 102mg of copper nitrate trihydrate, and 170ml of deionized water into a 250ml volume round-bottomed flask, put it in an ultrasonic machine and mix well to obtain Mixture A. The mixture A was placed in an oil bath for heating and reflux, the stirring speed was 725 rpm, and the temperature of the oil bath was set to 118 o C, reacted for 12.5h, cooled to room temperature naturally after the reflux reaction, and obtained mixed solution B after ultrasonic uniformity;
[0035](2) Transfer the mixed solution B into the flask used for rotary evaporation, and perform the rotary evaporation operation. The temperature of the rotary evaporator is controlled to 52 o C, the rotating speed is set to 38 rpm, after 2.2 hours of rotary evaporation, the solvent is completely evaporated, and the gray solid is fully separated from the mixed solution. Remove the flask used f...
Embodiment 2
[0038] Preparation of BCN-Cu single-atom catalyst
[0039] (1) Add 19.95g of urea, 2.01g of PEG-2000, 602mg of boric acid, 100mg of copper nitrate trihydrate, and 180ml of deionized water into a 250ml volume round-bottomed flask, put it in an ultrasonic machine and mix well to obtain mixed solution A. The mixed solution A was placed in the oil bath pot for heating and refluxing, the stirring speed was 750 rpm, and the oil bath pot temperature was set to 121 o C, react for 12h, naturally cool to room temperature after the reflux reaction, and obtain mixed solution B after ultrasonic uniformity;
[0040] (2) Transfer the mixed solution B into the flask used for rotary evaporation, and perform the rotary evaporation operation. The temperature of the rotary evaporator is controlled to 49 o C, the rotating speed is set to 41 rpm, after rotary evaporation for 2 hours, the solvent is completely evaporated, and the gray solid is fully separated from the mixed solution. Remove the f...
Embodiment 3
[0043] Preparation of BCN-Cu single-atom catalyst
[0044] (1) Add 13.85g of cyanamide, 1.98g of PEG-2000, 605mg of boric acid, 97mg of copper nitrate trihydrate, and 170ml of deionized water into a 250ml volume round-bottomed flask, put it in an ultrasonic machine and mix thoroughly to obtain a mixed solution A . The mixed solution A was placed in an oil bath for heating and reflux, the stirring speed was 800 rpm, and the temperature of the oil bath was set to 125 rpm. o C, react for 11.5h, naturally cool to room temperature after the reflux reaction, and obtain mixed solution B after sonicating uniformly;
[0045] (2) Transfer the mixed solution B into the flask used for rotary evaporation, and perform the rotary evaporation operation. The temperature of the rotary evaporator is controlled to 50 o C, the rotating speed is set to 42 rpm, and after 2 hours of rotary evaporation, the solvent is completely evaporated, and the dark gray solid is fully separated out from the mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com