Five pancreatic lipase inhibitors derived from small red bean protein and application thereof
A pancrelipase and inhibitor technology, applied in peptide/protein components, medical preparations containing active ingredients, metabolic diseases, etc., can solve problems such as sudden death, achieve good lipid-lowering effect, clear target, and clear mechanism of action Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Extraction of adzuki bean protein
[0022] The adzuki bean protein was extracted by the method of alkali dissolution and acid precipitation. Specifically, defatted adzuki bean powder and distilled water were mixed at a solid-liquid ratio of 1:10 (w / v), and the pH of the solution was adjusted to 8.5. After shaking in a water bath for 1 h at 40°C, the supernatant was collected, and after the pH value of the supernatant was adjusted to 4.5, the protein was allowed to stand for 1 h at room temperature to precipitate the protein. The precipitate was collected, washed 3 times with distilled water, and the pH of the protein was adjusted to 7.0. Finally, freeze-dry and store at -20°C.
Embodiment 2
[0023] Example 2 In vitro simulated digestion
[0024] Adzuki bean protein was hydrolyzed by double-enzyme (pepsin and trypsin) method to obtain protein hydrolyzate. Specifically, 4% (w / w) pepsin was first added to 5% adzuki bean protein solution, the pH of the enzymatic hydrolysis was 2.0, the enzymatic hydrolysis temperature was 37 °C, and the enzymatic hydrolysis time was 2 h; then the pH of the enzymatic hydrolysis solution was The pH value was adjusted to 5.3, the pH value was maintained at 7.5, 4% trypsin (w / w) was added, the enzymatic hydrolysis temperature was 37°C, and the enzymatic hydrolysis time was 2 h. After the enzymatic hydrolysis, the enzyme was inactivated in a boiling water bath for 10 min, and the enzymatic hydrolysis solution was centrifuged to take the supernatant.
Embodiment 3
[0025] Example 3 Screening of lipid-lowering peptides
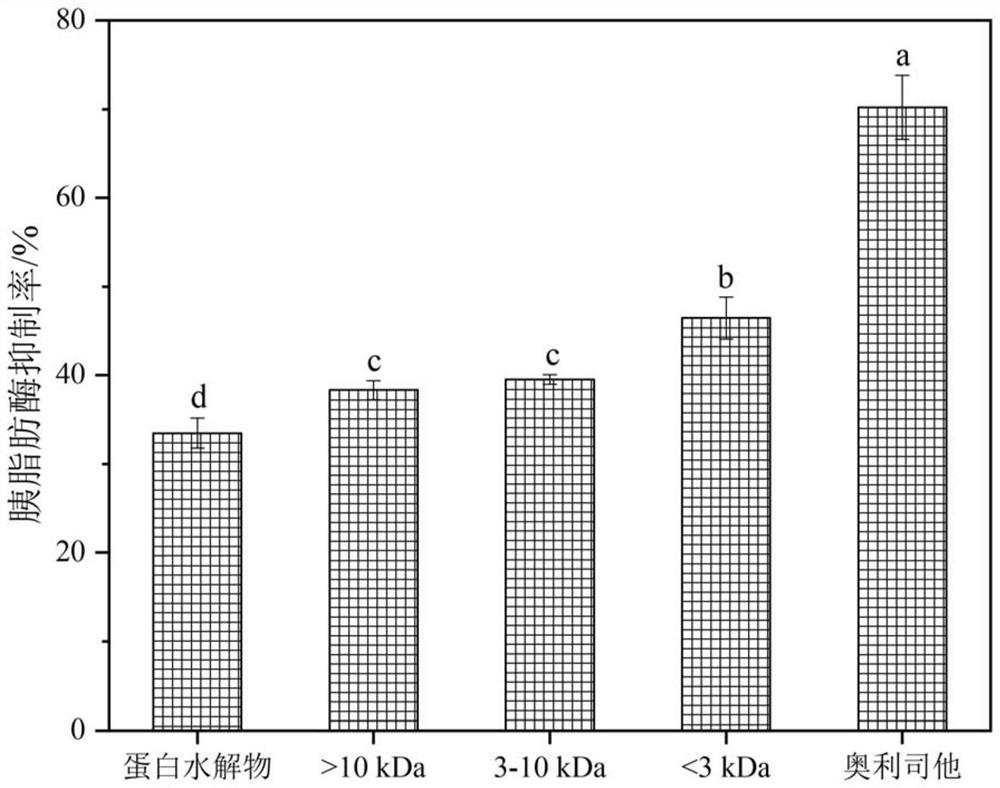
[0026] The supernatant collected in step (2) was ultrafiltered by using 10 kDa and 3 kDa centrifugal filters to obtain different fractions (>10 kDa, 3-10 kDa and figure 1 ). The specific process of the pancreatic lipase activity inhibition experiment is as follows:
[0027] In a 96-well microtiter plate, 50 μL of sample, 40 μL of 2.5 mg / mL pancrelipase solution and 50 μL of 10 mM p-nitrophenyl butyrate were used as substrates and incubated in phosphate buffer pH 7.3 for 30 min at 37 °C . The microplate reader records the absorbance at 405 nm. Orlistat was used as a positive control and calculated according to formula (1).
[0028]
[0029] In formula (1): A: the absorbance of the control; B: the absorbance of the control blank; C: the absorbance of the sample; D: the absorbance of the sample blank.
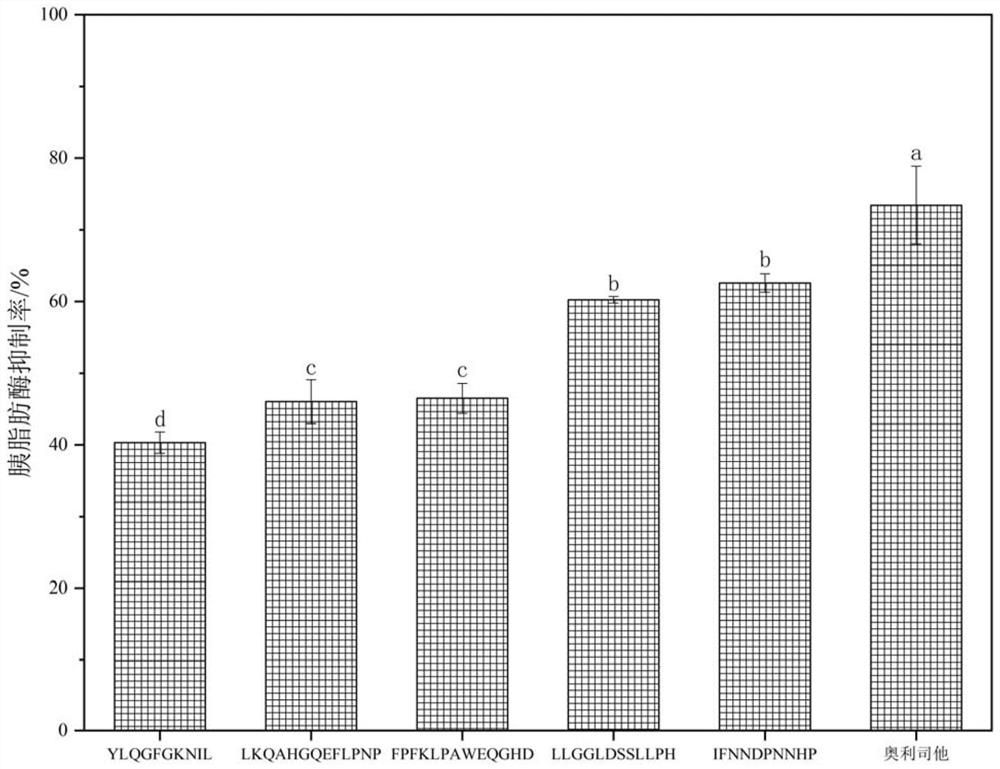
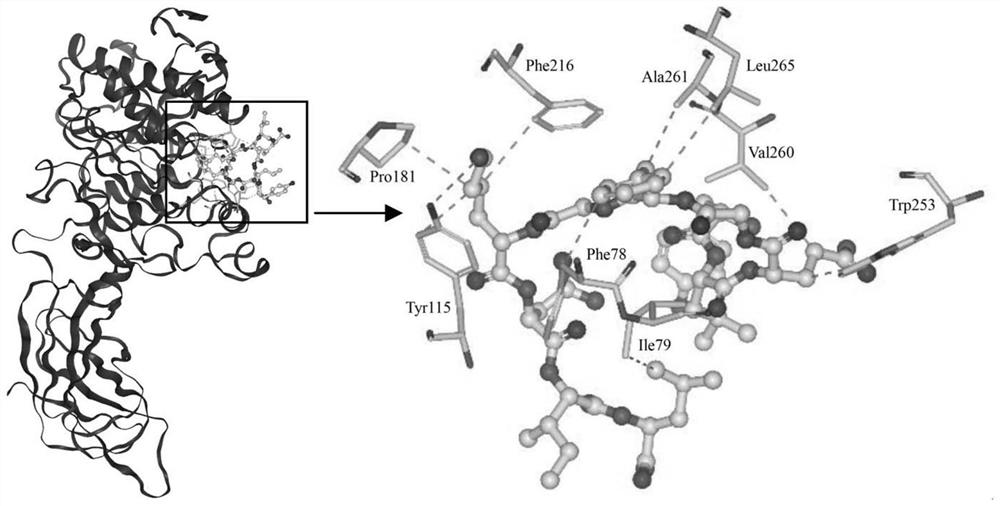
[0030] The <3kDa fractions were sequenced by mass spectrometry using liquid chromatography-tandem mass spectrometry,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com