Method for improving catalytic hydrogen production performance of copper-based catalyst
A technology of copper-based catalysts and performance, applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of synergy between catalyst acid function and reforming function, high price level of dual-function catalysts, etc. , to achieve the effect of low material price, simple method and shortened pitch
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Preparation of acid-activated montmorillonite
[0048] Disperse 6 g of montmorillonite in 294 g of nitric acid solution with a mass fraction of 20%, stir in an oil bath at 80 °C for 12 hours, and then use multiple centrifugations to wash the solution to neutrality, and then centrifuge the solid. Put it in an oven at 100°C to dry to constant weight, then grind the dried solid into powder, put it into a muffle furnace, and heat it up to 550°C for 4 hours at a heating rate of 5°C / min to obtain acid-activated montmorillonite. earth.
[0049] 2. Preparation of acid-activated montmorillonite supported copper-based catalyst
[0050] Using the excessive impregnation method, according to the loading of metal Cu as 10% of the mass of the carrier, 0.5875 g of copper nitrate was dissolved in 20 g of water, 2 g of acid-activated montmorillonite was added to it, stirred for 30 minutes, ultrasonicated for 30 minutes, and aged at room temperature After 12 hours, the excess water w...
Embodiment 2
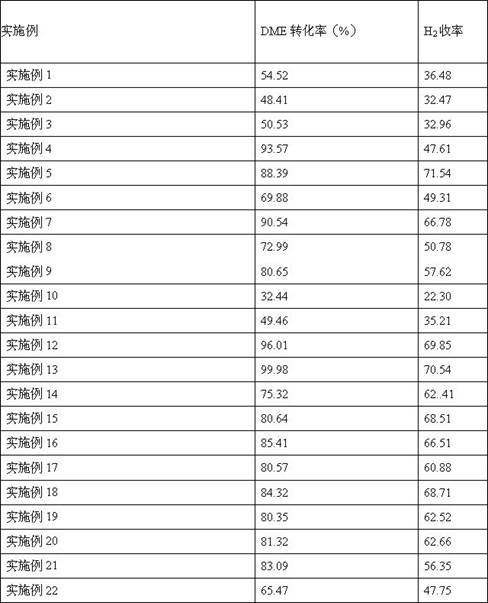
[0052] In step 2 of preparing acid-activated montmorillonite-supported copper-based catalyst in Example 1, 0.2938 g of copper nitrate was dissolved in 20 g of water, and 2 g of acid-activated montmorillonite was added to it. Other steps in this step were the same as those in Example 1. Similarly, an acid-activated montmorillonite-supported copper-based catalyst was prepared, wherein the loading of Cu was 5% of the mass of the support. The catalyst was used according to the method of use, and the reaction results are shown in Table 1.
Embodiment 3
[0054] In step 2 of preparing acid-activated montmorillonite-supported copper-based catalyst in Example 1, 0.8813 g of copper nitrate was dissolved in 20 g of water, and 2 g of acid-activated montmorillonite was added to it. Other steps in this step were the same as those in Example 1. In the same way, an acid-activated montmorillonite-supported copper-based catalyst was prepared, wherein the loading of Cu was 15% of the mass of the support. The catalyst was used according to the method of use, and the reaction results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size distribution | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com