Novel formulations and methods
A composition and nanoparticle technology, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, inorganic non-active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] The preparation method of the composition of the present invention
[0107] In one embodiment, T3 is covalently attached to the biodegradable polymer, eg, through a hydroxyl group on the phenyl moiety. Such compositions can be formed using activated T3 that is activated with a suitable linker at the phenolic hydroxyl group and the amino moiety is protected. For example, in one embodiment, amino protected T3 is formed using the synthesis shown in Scheme 1 below.
[0108] plan 1:
[0109]
[0110] The amino-protected T3 is then attached to the nanoparticle, eg, via a phenolic hydroxyl group, eg, by using an activated linking group, eg, an amino group capable of coupling to a bioabsorbable polymer, eg, chitosan the amino moiety.
[0111] Thus, in one embodiment, the present invention provides a compound of the formula [CH2-O-CH]-[CH2] on its phenolic hydroxyl group n - Activated T3 substituted with an epoxy group and protected by an amino group. For example, the pr...
Embodiment 1
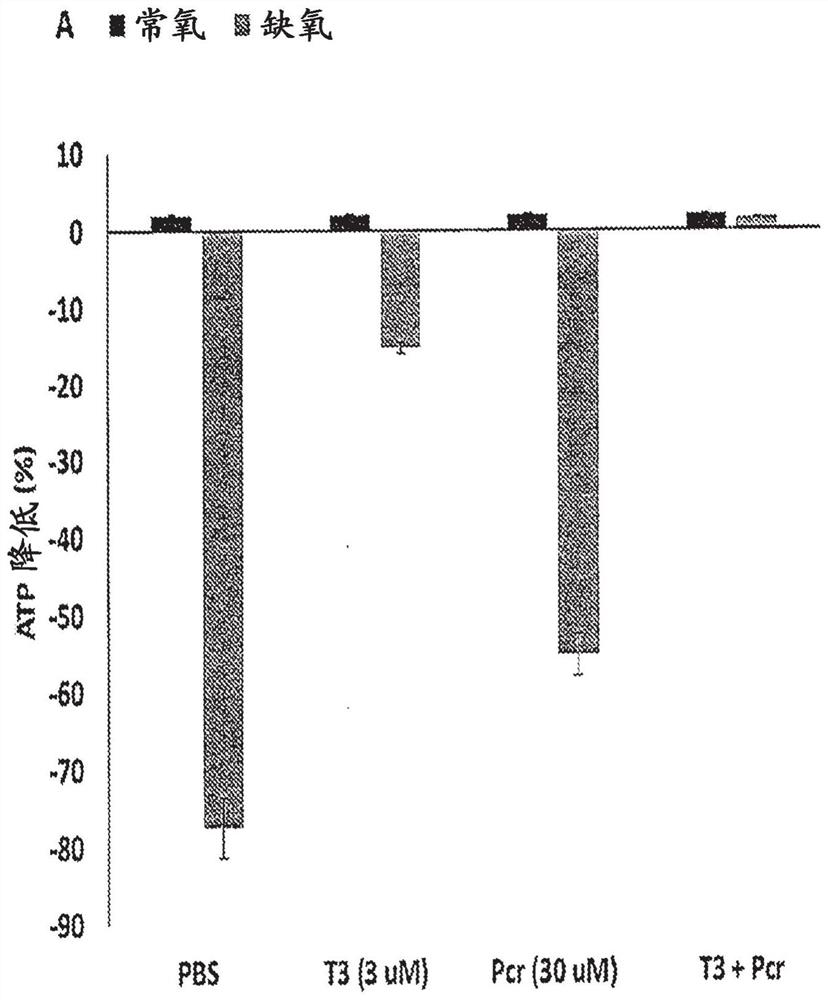
[0134] Example 1: Cardiovascular protection of T3 and phosphocreatine nanoparticles on ischemic injury
[0135] This study aimed to investigate the active protective role of T3, T3 nanoparticles, and T3 and phosphocreatine nanoparticles (PCR) in hypoxia-mediated cardiomyocyte injury, and the effect of vascularization and neuronal protection under hypoxia. influences. The effect of T3 and T3 nanoparticles + PCR on angiogenesis was investigated in the CAM model. The cardioprotective effects of T3 under hypoxia were investigated by treating isolated neonatal cardiomyocytes with PBS (control group), free T3 (3 μm), free PCR (30 μm), and T3 nanoparticles + PCR. Mitochondrial function and sarcomere integrity were measured by ATP-bioluminescence and flow cytometry, respectively. The effect of T3 nanoparticles on hypoxia-induced neuronal cells was also investigated. Finally, T3 and T3 nanoparticles labeled with Cy7 dye were injected into the tail vein of mice to monitor their biodi...
Embodiment 2
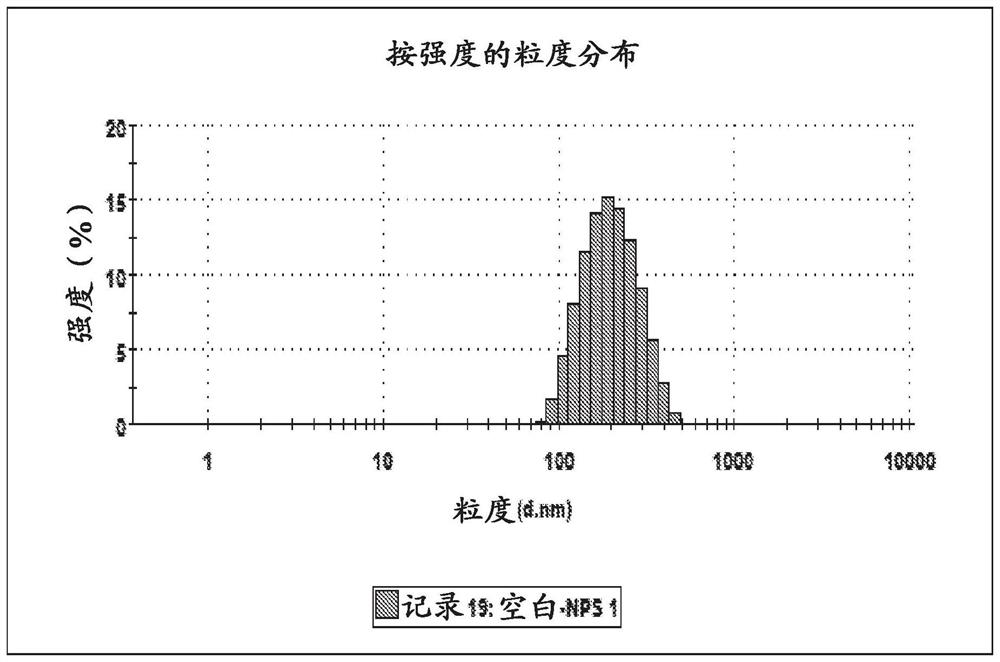
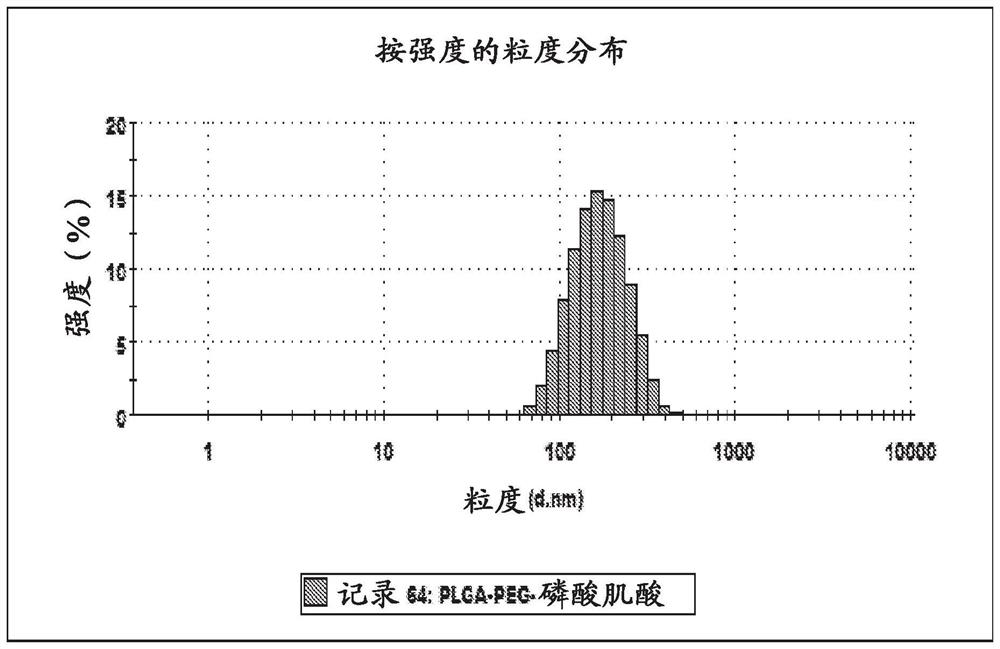
[0138] Example 2: Synthesis and size comparison of different nanoparticle matrices
[0139] Several different potential polymer matrices were created for nanoparticles. T3-containing PLGA-based nanoparticles were prepared by dissolving 200 mg of PLGA and 20 mg of T3 in 1 mL of dimethyl sulfoxide. Under sonication, the solution was added dropwise to 40 mL of 1% PVA. The emulsion was lyophilized and the DMSO removed. The product was then reconstituted in 20 mL of PBS.
[0140] A similar approach was used to prepare PLGA-based nanoparticles containing T3 and PCR. PLGA-based nanoparticles containing T3 were prepared by dissolving 250 mg PLGA and 25 mg T3 in 1 mL DMSO. The solution was added dropwise to 20 mL of 1% PVA solution containing 45 mg PCR under sonication. The resulting emulsion was then added dropwise to 30 mL of 1% PVA. The emulsion was lyophilized and reconstituted in 25 mL of PBS.
[0141] PLGA-PEG-based nanoparticles were also prepared. The mixture of PLGA-PE...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com