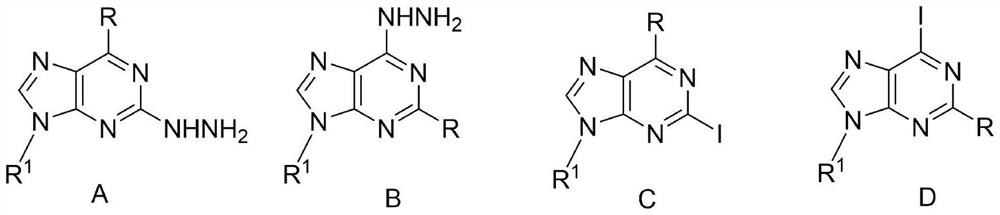
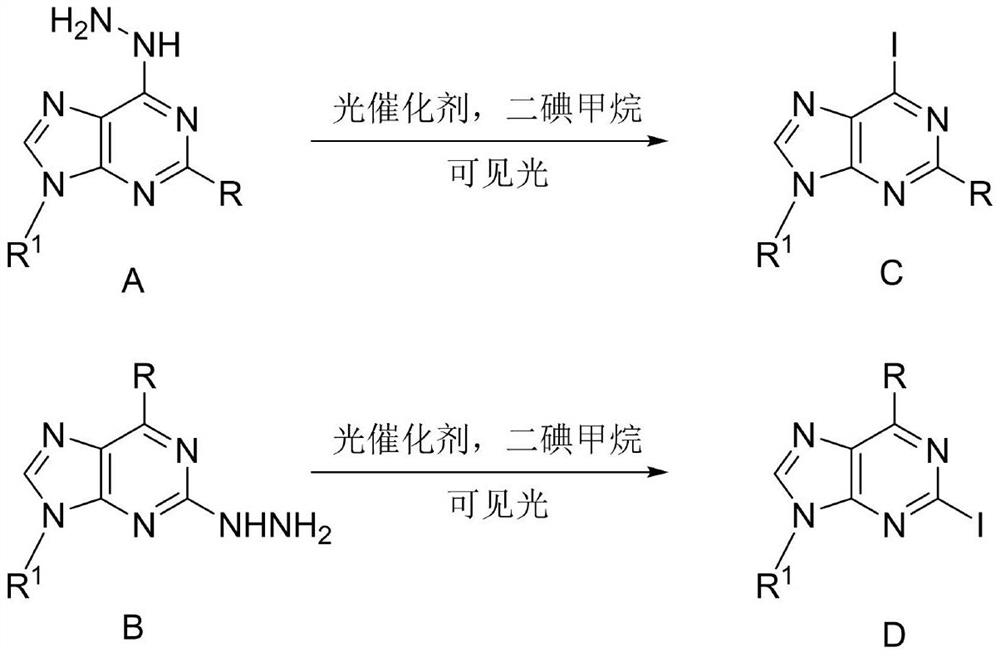
Method for synthesizing 2/6-iodo-purine derivative through visible light induced hydrazine removal-iodination reaction
A technology of derivatives, iodopurine, is applied in the field of visible light-induced dehydrazine-iodination reaction to synthesize 2/6-iodopurine derivatives, can solve problems such as narrow application range, and achieve wide applicability, simple operation and safety factor high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a transparent glass reaction flask, add (0.24g, 1mmol), diiodomethane (0.088mL, 1.1mmol) and eosin Y (0.0143g, 0.02mmol) were added to acetonitrile (2mL), turned on 5W visible light, stirred, irradiated at room temperature for 4h, TLC showed The raw materials reacted completely, the reaction solution was concentrated under reduced pressure, and separated by column chromatography to obtain 0.299g, yield 89%.
[0025] 1 H NMR (400MHz, DMSO-d 6 )δ8.78(s,1H),8.13(s,1H),7.37-7.31(m,5H),5.47(s,2H); 13 C NMR (100MHz, DMSO-d 6 )δ152.1,151.8,151.0,134.5,131.5,129.2,128.8,127.9,68.7,47.8.
Embodiment 2
[0027] by As the raw material, other reaction conditions were changed, and the reaction results were shown in the following table:
[0028]
Embodiment 3
[0030] In a transparent glass reaction flask, add (0.297g, 1mmol), diiodomethane (0.081mL, 1mmol) and eosin Y (0.0143g, 0.02mmol) were added to acetonitrile (5mL), turned on 5W visible light, stirred, irradiated at room temperature for 6h, TLC showed the raw materials The reaction was complete, the reaction solution was concentrated under reduced pressure, and recrystallized from ethanol to obtain 0.294g, yield 75%.
[0031] 1 H NMR (400MHz, DMSO-d 6 )δ8.15(s,1H),7.37(brs,2H,NH 2 ),5.89(d,J=6.4Hz,1H),5.47(t,J=6.4Hz,2H),5.23(d,J=4.8Hz,1H),4.64-4.60(m,1H),4.17-4.14 (m, 1H), 3.99-3.96 (m, 1H), 3.70-3.66 (m, 1H), 3.59-3.53 (m, 1H); 13 C NMR (100MHz, DMSO-d 6 )δ152.5,149.4,140.4,118.3,84.1,83.6,75.7,75.0,68.2,60.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com