Axial chiral solid fluorescent material with helicene structure
A solid-state fluorescence and axial chiral technology, applied in the field of fluorescent materials, can solve the problems of low luminous efficiency of solid-state fluorescence and limit the practical application of chiral organic light-emitting materials, and achieve high absolute fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. Synthesis of Compounds (R)-BINOL[4]helicene-1 and (S)-BINOL[4]helicene-1
[0026] The reaction formula is as follows:
[0027]
[0028] 1) Add 3.2g (7.95mmol) (R)-2,2'-bis(methoxymethoxy)-[1,1'-binaphthyl]- to a 100ml round bottom flask under ice bath condition 3-aldehyde, 4.4 g (8.57 mmol) 4-bromobenzyltriphenylphosphine bromide, and 40 ml dry tetrahydrofuran. After the reactants were completely dissolved, 5.9 g (61.4 mmol) of sodium tert-butoxide was added to the reaction solution in batches, and the reaction was carried out under ice bath conditions for 4 h. Water was added to quench the reaction, dichloromethane was added to the reaction solution, the organic phase obtained by separation was dried with anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation, and 4.1 g of (R,E)-3-( 4-Bromostyryl)-2,2'-bis(methoxymethoxy)-1,1'-binaphthyl, 95% yield.
[0029] 2) Under nitrogen protection, add 0.2g (0.36mmol) (R,E)-3-(4-bro...
Embodiment 2
[0034] Example 2. Synthesis of Compounds (R)-BINOL[4]helicene-2 and (S)-BINOL[4]helicene-2
[0035] The reaction formula is as follows:
[0036]
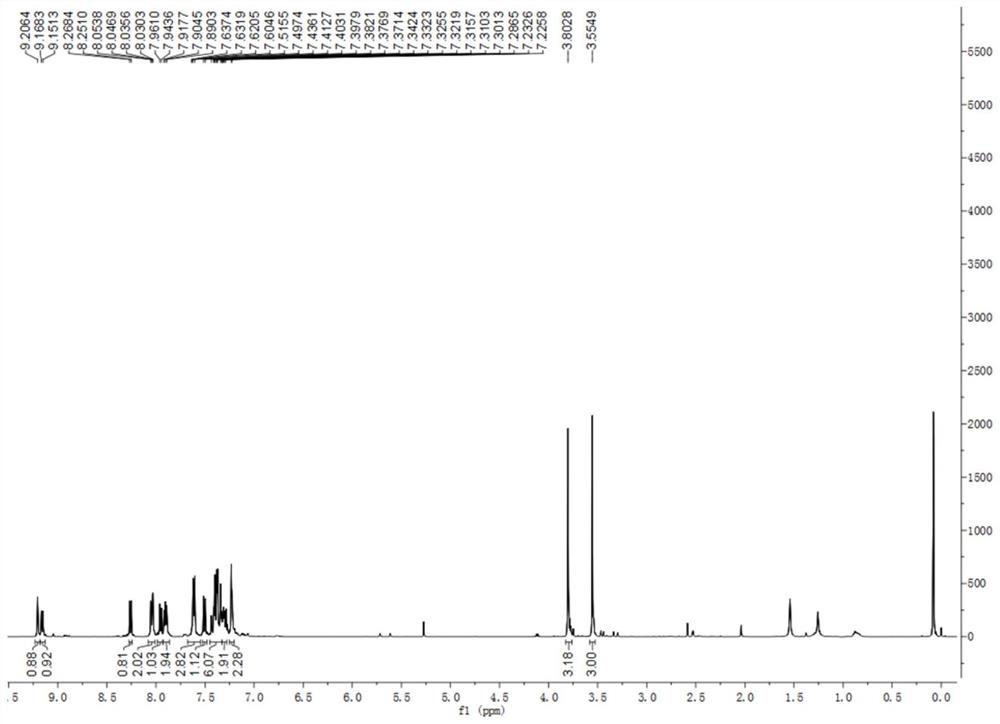
[0037] Under nitrogen protection, add 300mg (0.60mmol) (R)-2-bromo-7-methoxy-8-(2-(methoxy)naphthalen-1-yl)benzo[c] to a 100ml three-necked flask phenanthrene, 24 mg (0.11 mmol) palladium acetate and 82.5 mg (0.27 mmol) tri-o-methylphenylphosphine, then 25 ml dry DMF and 20 ml triethylamine were added. The solution was heated to 45° C. for 0.5 h, and 93 mg (0.72 mmol) of 4-vinyl benzonitrile was added. Then the solution was warmed to 115°C and reacted for 5h. DMF and triethylamine were removed by rotary evaporation, and dichloromethane was added to the solution. The organic phase was washed with water, spin-dried, and separated by column chromatography to obtain 0.19 g of (R, E)-4-(2-(7-methoxy-8-(2-methoxynaphthalen-1-yl)benzo[c] ]phenanthren-2-yl)vinyl)benzonitrile (R)-BINOL[4]helicene-2 in 64% yield. 1 HNMR (500MHz, C...
Embodiment 3
[0040] Example 3. Synthesis of Compounds (R)-BINOL[4]helicene-3 and (S)-BINOL[4]helicene-3
[0041] The reaction formula is as follows:
[0042]
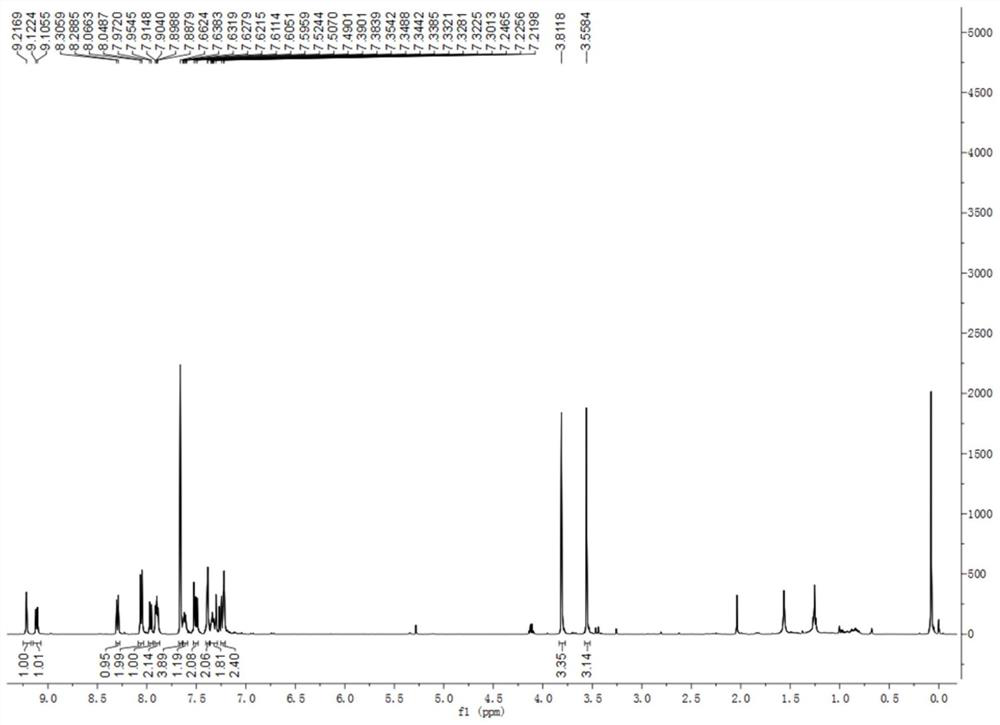
[0043] Under nitrogen protection, add 300mg (0.60mmol) (R)-2-bromo-7-methoxy-8-(2-(methoxy)naphthalen-1-yl)benzo[c] to a 100ml three-necked flask phenanthrene, 24 mg (0.11 mmol) palladium acetate and 82.5 mg (0.27 mmol) tri-o-methylphenylphosphine, then 25 ml dry DMF and 20 ml triethylamine were added. The solution was heated to 45° C. for 0.5 h, and 97 mg (0.72 mmol) of 4-methoxystyrene was added. Then the solution was warmed to 115°C and reacted for 5h. DMF and triethylamine were removed by rotary evaporation, and dichloromethane was added to the solution. The organic phase was washed with water, spin-dried, and separated by column chromatography to obtain 0.20 g of (R, E)-7-methoxy-8-(2-methoxynaphthalen-1-yl)-2-(4-methoxybenzene) Vinyl)benzo[c]phenanthrene(R)-BINOL[4]helicene-3, 62% yield. 1 HNMR (500MHz, CDCl 3 )δ9.17(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com