Methods of treating lupus nephritis using interleukin-17 (IL-17) antagonists
A lupus nephritis and antibody technology, applied in antiviral agents, chemical instruments and methods, antibodies, etc., can solve the problems of lack of treatment and unmet medical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
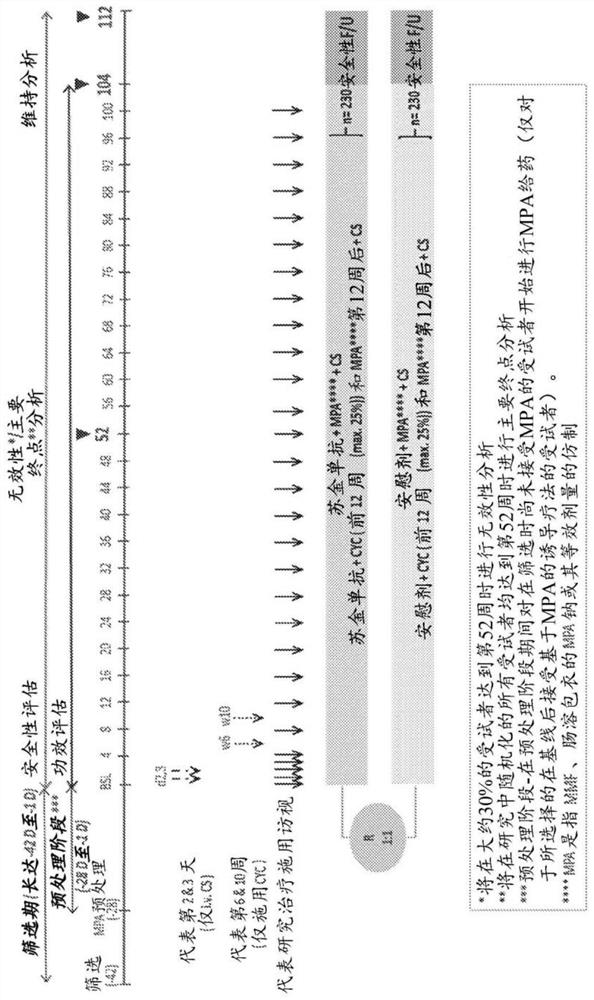
[0203] A two-year, phase III randomized, double-blind, parallel-group, placebo-controlled trial to evaluate 300 mg s.c. secukinumab (versus placebo) in combination with SoC therapy in patients with active lupus nephritis safety, efficacy and tolerability.
[0204] Research purposes
[0205] The purpose of this trial was to evaluate subcutaneous (SC) administration of secukinumab 300 mg (compared to placebo) in combination with standard of care therapy (SoC) in patients with active lupus nephritis (ISN / RPS class III or IV, with Efficacy and safety in subjects without coexisting Class V characteristics).
[0206] The background SoC will consist of the following: with mycophenolic acid (MPA) (referring to mycophenolate mofetil or general equivalent) or at equivalent doses (oral) enteric-coated sodium MPA ( or general equivalent), or induction therapy with cyclophosphamide (CYC) (i.v.), followed by maintenance therapy with MPA. In addition, all subjects will receive i.v. and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com