Safe and effective method of treating psoriasis with Anti-il-23 specific antibody
A psoriasis, antibody technology, applied in the direction of antibody medical ingredients, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
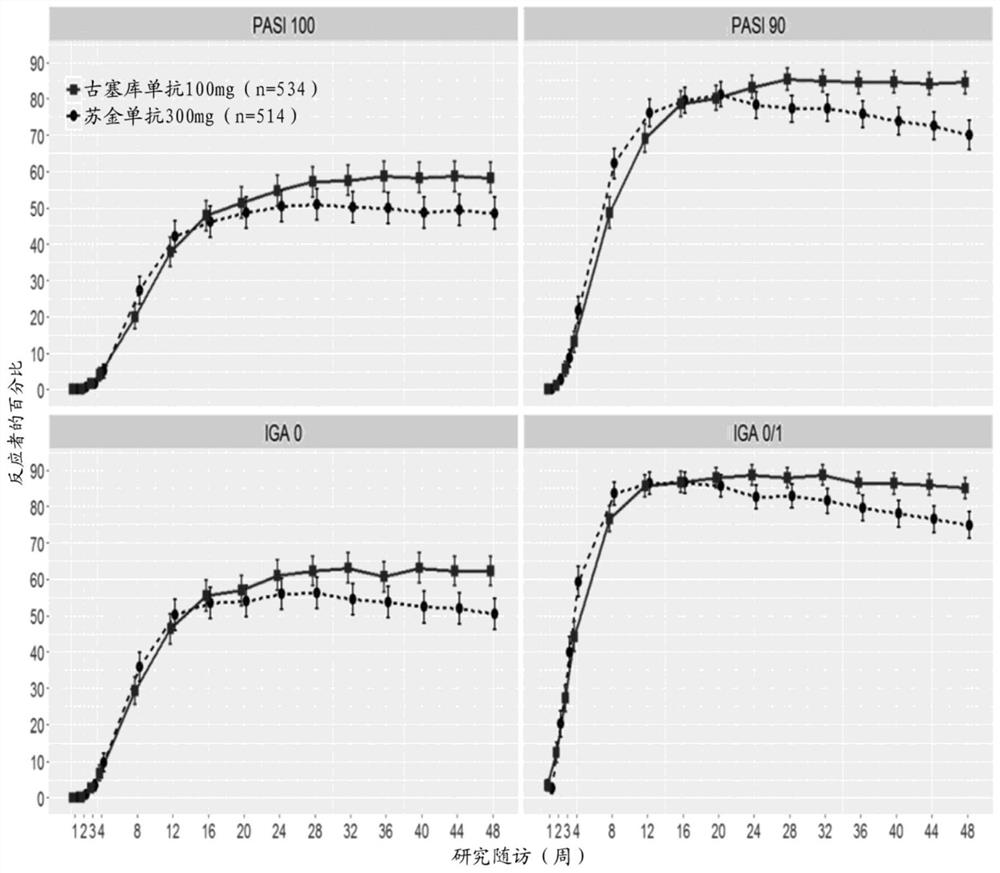
[0219] Example 1: Evaluation of CNTO 1959 (guselkumab) and secukinumab for the treatment of moderate to severe plaque silver A phase 3 multicenter randomized double-blind study of comparative efficacy in psoriasis
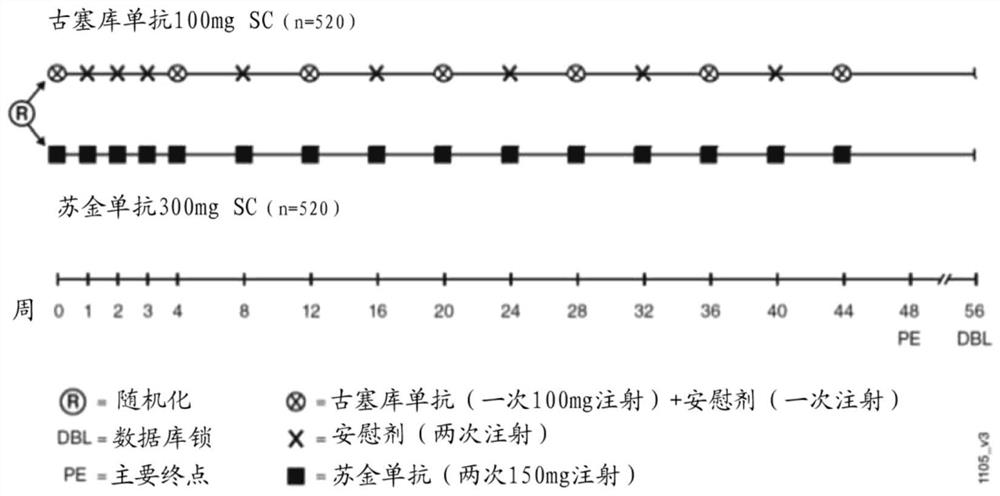
[0220] Research design :
[0221] ●Phase 3 randomized double-blind multicenter active comparator study in subjects with moderate to severe plaque psoriasis including 2 parallel treatment arms: guselkumab 100 mg and secukinumab 300mg.
[0222] Randomization: At week 0, approximately 1040 subjects meeting all inclusion and exclusion criteria are planned to be randomized in a 1:1 ratio to the 2 arms based on permuted block randomization using stratification by study site 1 of:
[0223] ○ Group I (n=520): Guselkumab, 100 mg, SC, administered at weeks 0, 4, 12, 20, and then every 8 weeks until week 44.
[0224] ○ Group II (n=520): secukinumab, 300 mg, SC, administered at weeks 0, 1, 2, 3, and 4, and then every 4 weeks until week 44.
[0225] ●Treatment Duratio...
Embodiment 2
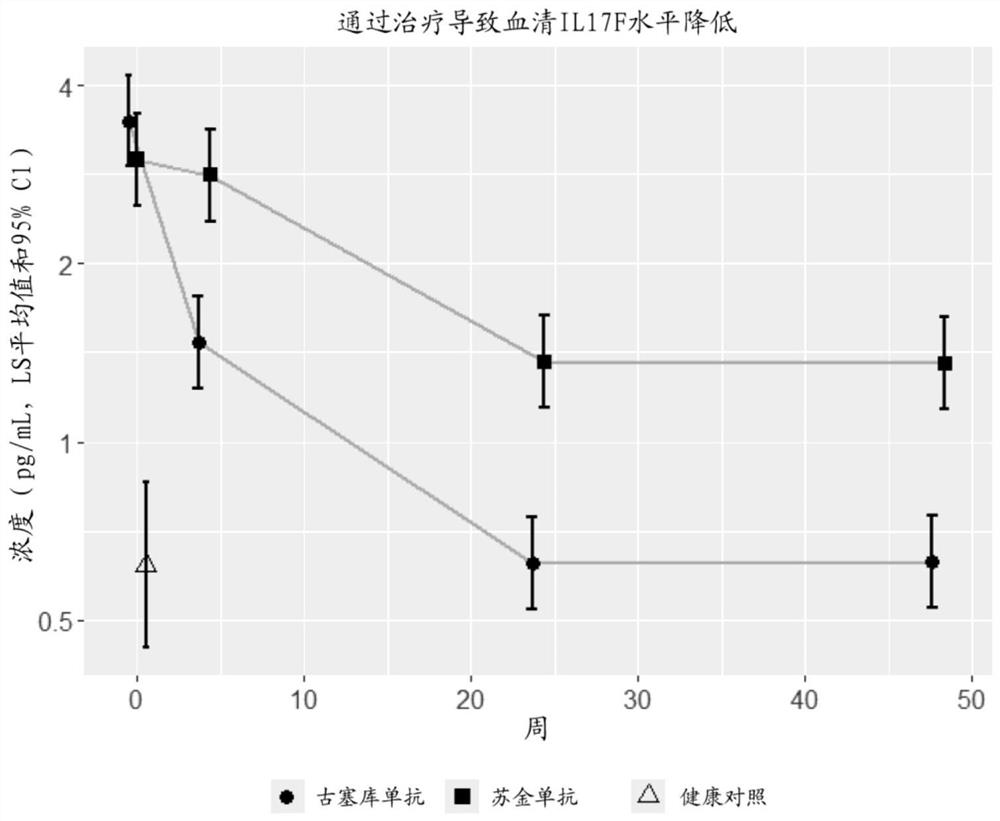
[0301] Example 2 - Evaluation of anti-IL-23 and anti-IL-17A on IL-17F levels and IL-17F levels of immune cell populations in skin and serum Effect of treatment on IL-22 levels
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com