Method for co-producing alcohol and hydrogen through chemical chain conversion of natural gas hydrate
A technology of natural gas and physical chemistry, applied in chemical instruments and methods, physical/chemical process catalysts, bulk chemical production, etc., to achieve the effects of high comprehensive resource utilization efficiency, wide application scenarios, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
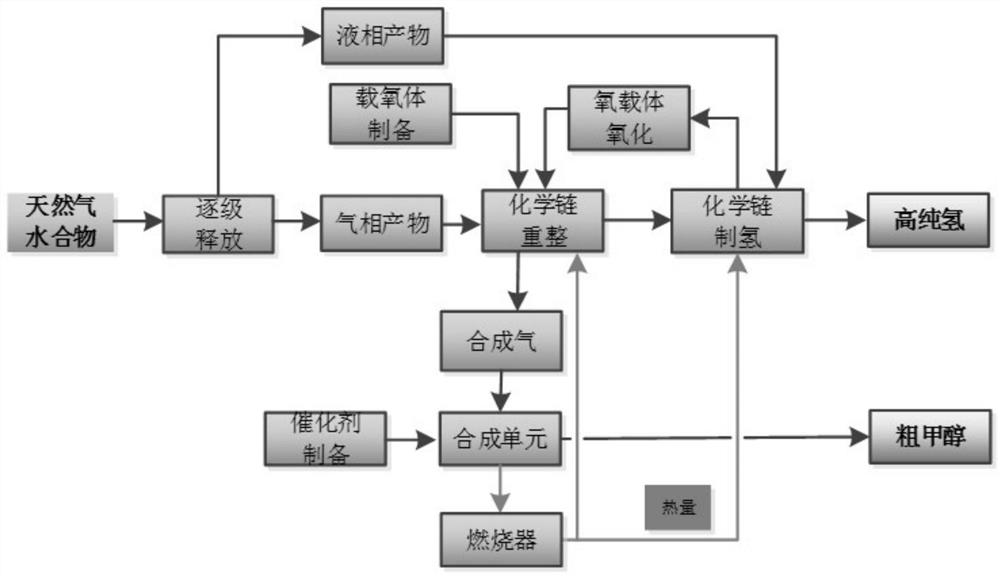
[0027] The present embodiment discloses a method for co-producing alcohol hydrogen by chemical chain conversion of natural gas hydrate, comprising the following steps:
[0028] (1) Natural gas hydrate is released into gas-phase products and liquid-phase products through three-stage decompression at 6MPa, 2MPa, and 1MPa (temperature range is -160°C to normal temperature), reducing heat loss and realizing gas-liquid separation.
[0029] (2) The Fe / Ni / Al-based composite oxygen carrier precursor was constructed by chemical precipitation, and then calcined at 1000 °C for 3 hours, and then crushed and screened to prepare oxygen carrier particles with a particle size ranging from 60 to 80 meshes.
[0030] (3) The gas phase product obtained in step (1) and the Fe / Ni / Al (atomic ratio 0.6 / 0.2 / 0.2) based composite oxygen carrier obtained in step (2) are subjected to a chemical chain reforming process to prepare syngas, and the reaction temperature is 850°C, the reaction residence time wa...
Embodiment 2
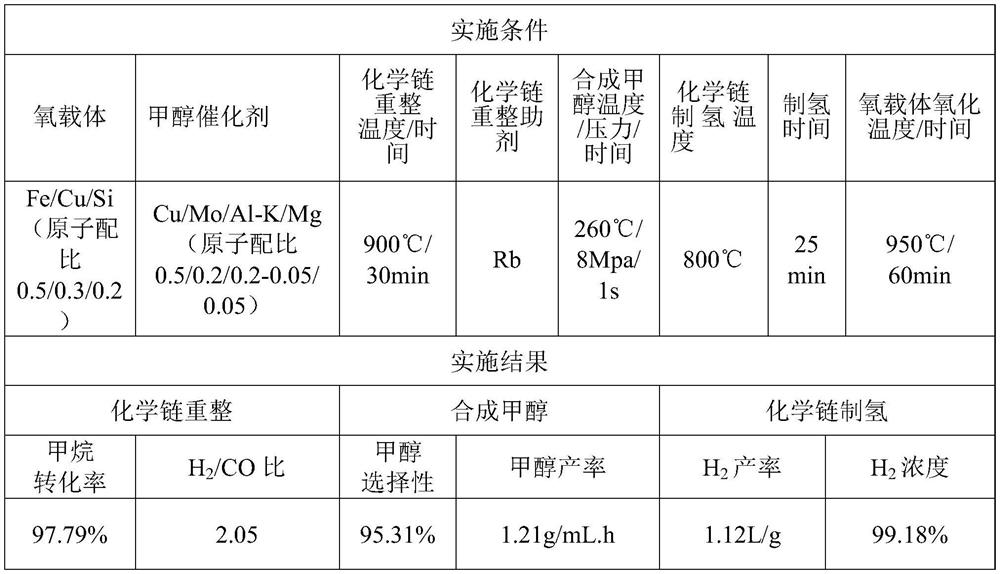
[0038] Since the method of chemical chain conversion of natural gas hydrate for co-production of alcohol and hydrogen is the same as that in Example 1, it will not be repeated here, only the implementation conditions are listed in Table 1, and the implementation results are shown in Table 1.
[0039] Table 1 Example of chemical chain conversion of natural gas hydrate for co-production of alcohol hydrogen
[0040]
[0041] In this example, the Fe / Cu / Si-based composite oxygen carrier is constructed by the chemical chain self-assembly method, and then calcined at 950° C. for 4 hours, crushed and sieved to prepare oxygen carrier particles with a particle size ranging from 60 to 80 meshes. The preparation method of the synthetic methanol catalyst is the same as that in Example (1), and the active components and auxiliary agents used are indicated in the table.
Embodiment 3
[0043] Since the method for co-producing high-purity hydrogen by chemically chaining olefins from natural gas hydrate is the same as that in Example 1, it will not be repeated here, and only the implementation conditions and implementation results are listed in Table 2, as shown in Table 2.
[0044] Table 2 Examples of chemical chain conversion of natural gas hydrate for co-production of alcohol and hydrogen
[0045]
[0046]
[0047] The difference between Example 1 and Example 2 is that the oxygen carrier, the reaction temperature, the catalyst for synthesizing methanol and the reaction assistant are different. In Example 2, the methane conversion efficiency in the chemical chain reforming stage is improved, and the methanol selectivity in the methanol synthesis stage is good, In the hydrogen production stage, the hydrogen product concentration decreased. In Example 3, Fe / Mn / Ca oxygen carrier and Ce auxiliary agent are used to have better resistance to carbon depositio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com