Synthesis method of N-heterocyclic carbene fluorescent free radical compound
A nitrogen heterocyclic carbene and synthesis method technology, which is applied in the field of synthesis of nitrogen heterocyclic carbene fluorescent free radical compounds, can solve the problems of easy removal of chlorine atoms, single free radical structure, and low photon quantum yield, so as to avoid secondary Polymerization reaction, improved stability, and good universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
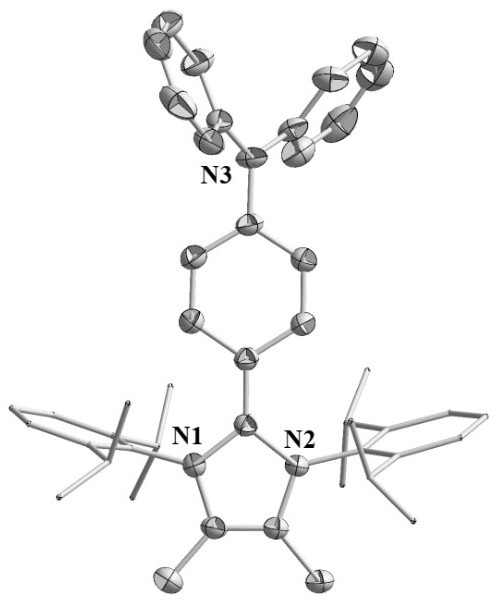
[0030] Example 1: Preparation of azacyclic carbene-triphenylamine radical 1a
[0031]
[0032] Step 1: Under a nitrogen atmosphere, the Pd 2 (dba) 3 18.3 mg (0.02 mmol), 4-iodo- N , N - Diphenylaniline 371.0 mg (1.0 mmol), azacyclic carbene a 416.5 mg (1.0 mmol) and 30 mL of dioxane were added to a 100 mL Schlenk tube to obtain a brown solution. The above mixture was first stirred at room temperature for 30 min, and then the temperature was raised to 120 o C reacted for 15 h. After the reaction was completed, it was cooled to room temperature and concentrated. Add n-hexane to the Schlenk tube, stir vigorously, filter and rinse with n-hexane, and dry to obtain 747.6 mg of azacyclic carbene-triphenylamine adduct as a gray product with a yield of 95%. Elemental Analysis (%), C 47 H 54 N 3 I (761.79): C 71.18, H 6.95, N 5.81; found: C 70.95, H6.68, N 5.70. 1 H NMR (400 MHz, CD 3 CN): 0.98 (d, J = 6.8 Hz, 12H, CH(C H 3 )), 1.22 (d, J = 6.8 Hz, 12H, CH(C H 3 )...
Embodiment 2
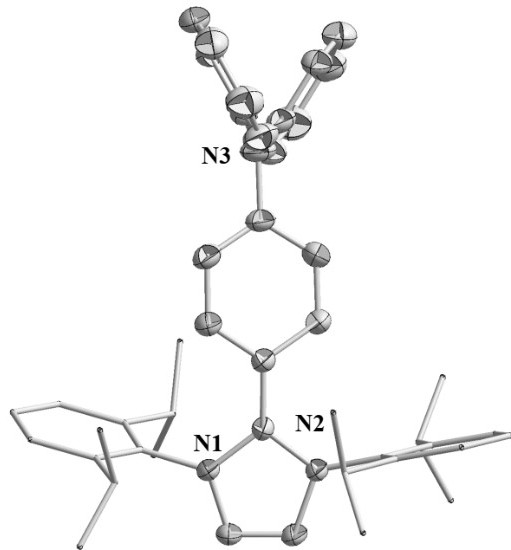
[0035] Example 2: Preparation of azacyclic carbene-triphenylamine radical 1b
[0036]
[0037] Step 1: Under a nitrogen atmosphere, the Pd 2 (dba) 3 18.3 mg (0.02 mmol), 4-iodo-N,N-diphenylaniline 371.0 mg (1.0 mmol), azacyclocarbene b 388.5 mg (1.0 mmol) and 30 mL dioxane were added to a 100 mL Schlenk tube A brown solution was obtained. The above mixture was first stirred at room temperature for 30 min, and then the temperature was raised to 120 o C reacted for 15 h. After the reaction was completed, it was cooled to room temperature and concentrated. Add n-hexane to a Schlenk tube, stir vigorously, filter and rinse the solid with n-hexane, and dry to obtain 577.4 mg of azacyclocarbene-triphenylamine adduct as a gray product with a yield of 76%. Elemental Analysis (%), C 45 H 50 N 3 I (759.78): C 71.46, H 6.91, N 5.81; found: C 71.14, H 6.63, N 5.53. 1 H NMR (400 MHz, CD 3 CN): 1.05 (d, 3 J H,H = 6.8 Hz, 12H, CH(C H 3 )), 1.23 (d, J = 6.8 Hz, 12H, CH(C ...
Embodiment 3
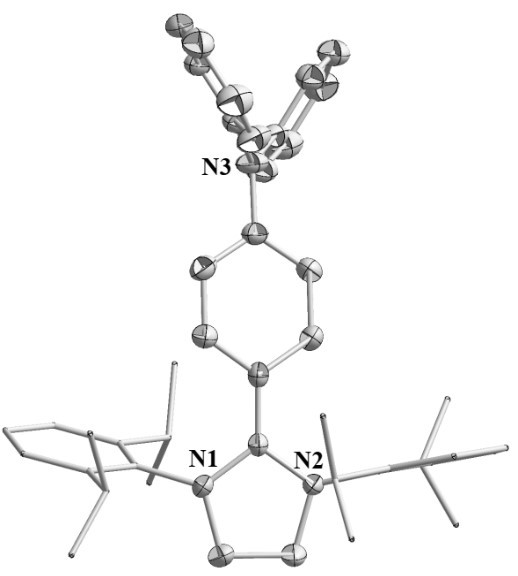
[0040] Example 3: Preparation of azacyclic carbene-triphenylamine radical 1c
[0041]
[0042] Step 1: Under a nitrogen atmosphere, the Pd 2 (dba) 3 18.3 mg (0.02 mmol), 4-iodo-N,N-diphenylaniline 371.0 mg (1.0 mmol), azacyclocarbene c 390.5 mg (1.0 mmol) and 30 mL dioxane were added to a 100 mL Schlenk tube A brown solution was obtained. The above mixture was first stirred at room temperature for 30 min, and then the temperature was raised to 120 o C reacted for 15 h. After the reaction was completed, it was cooled to room temperature and concentrated. Add n-hexane to the Schlenk tube, stir vigorously, filter and rinse the filter residue with n-hexane, and dry to obtain 646.8 mg of azacyclocarbene-triphenylamine adduct as a gray product with a yield of 85%. Elemental Analysis (%), C 45 H 54 N 3 I (761.79): C 71.18, H 6.95, N 5.81; found: C 70.95, H 6.68, N 5.70. 1 H NMR (400 MHz, CD 3 CN): 1.02 (d, J = 6.8 Hz, 12H, CH(C H 3 )), 1.33 (d, J = 6.8 Hz, 12H, CH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com