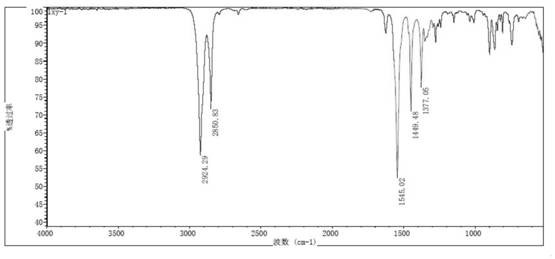
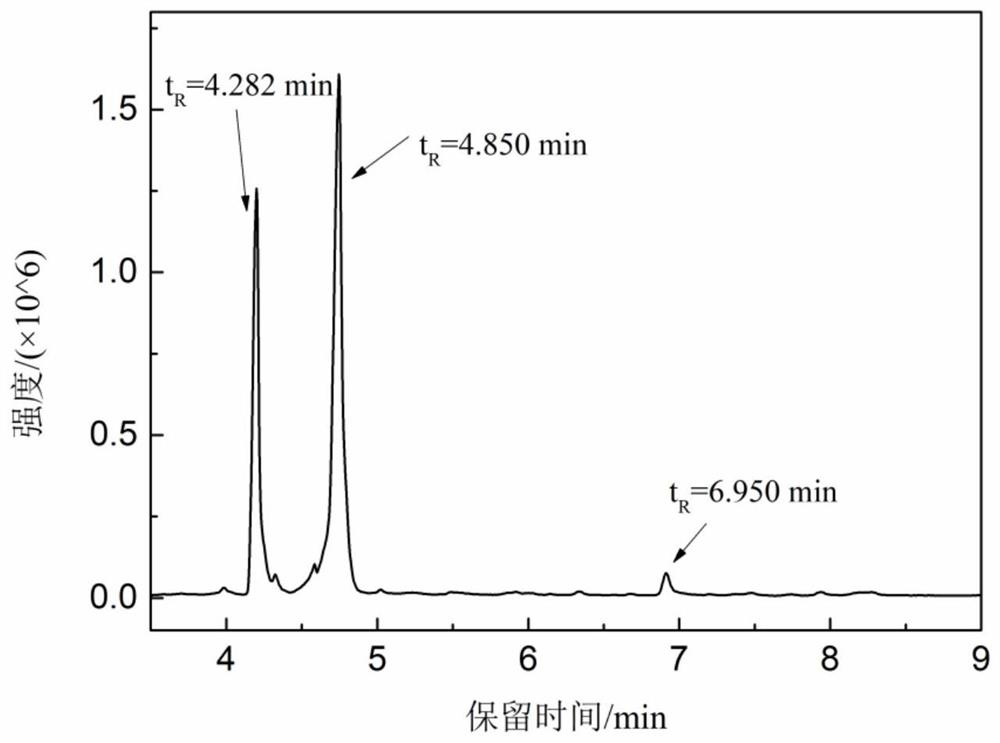
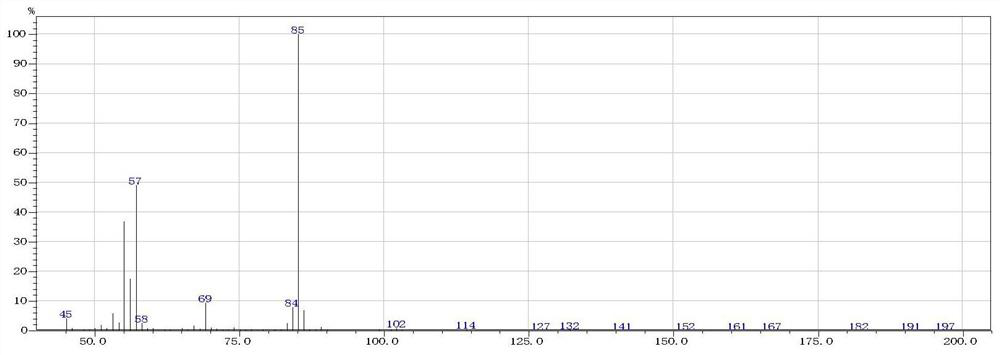
Preparation method of short-chain nitroalkane
A technology for nitroalkane and nitrohexane, which is applied in the field of chemical engineering, can solve the problems of difficult product separation, high reaction pressure and high reaction temperature, and achieves the advantages of cheap and easily available reaction raw materials, low reaction temperature and pressure, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 50ml micro-reactor, add n-hexane and NO successively 2 , n-hexane and NO 2 The ratio of the amount of substances is 1:1. The reaction temperature was set to 100°C and the reaction time was 4h. After the reaction, the reactor was cooled to room temperature, and then the reactor was safely opened to take out the reaction solution. A 5% aqueous sodium bicarbonate solution was added to the reaction solution to quench the reaction, and the reaction solution was washed with alkali until neutral, then washed with deionized water for several times, and finally dried with anhydrous sodium sulfate to remove water. Since the product nitrohexane is volatile, for the calculation of the yield, we directly take part of the reaction solution after simple treatment for gas chromatography and GC-MS detection and analysis. Under the reaction conditions, the conversion rate of n-hexane was 29.6%, and the selectivity of each product: 1-nitrohexane was 1.2%, 2-nitrohexane was 58.0%, ...
Embodiment 2
[0031] The process and the reactor used are the same as in Example 1, except that the reaction temperature is 120 ° C, and the conversion rate of n-hexane is recorded as 64.3% under the reaction conditions, and the selectivity of each product: the selection of 1-nitrohexane The selectivity was 1.4%, the selectivity of 2-nitrohexane was 57.6%, and the selectivity of 3-nitrohexane was 41.0%.
Embodiment 3
[0033] Process and used reactor are the same as in Example 1, except that n-hexane and NO 2 The ratio of the amount of the substances is 1:2, the conversion rate of n-hexane is 85.9% under this reaction condition, and the selectivity of each product: the selectivity of 1-nitrohexane is 2.0%, the selectivity of 2-nitrohexane is 2.0%, and the The selectivity of nitrohexane was 54.3%, and the selectivity of 3-nitrohexane was 43.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com