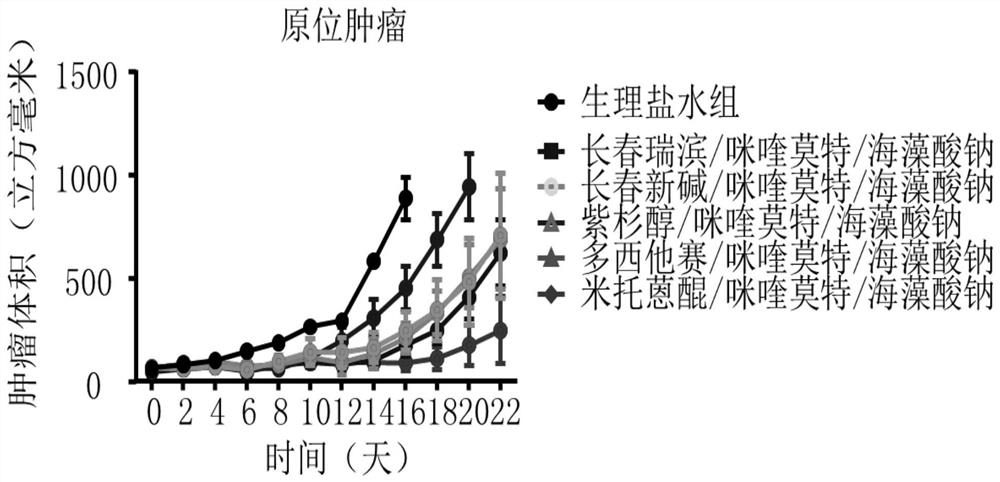
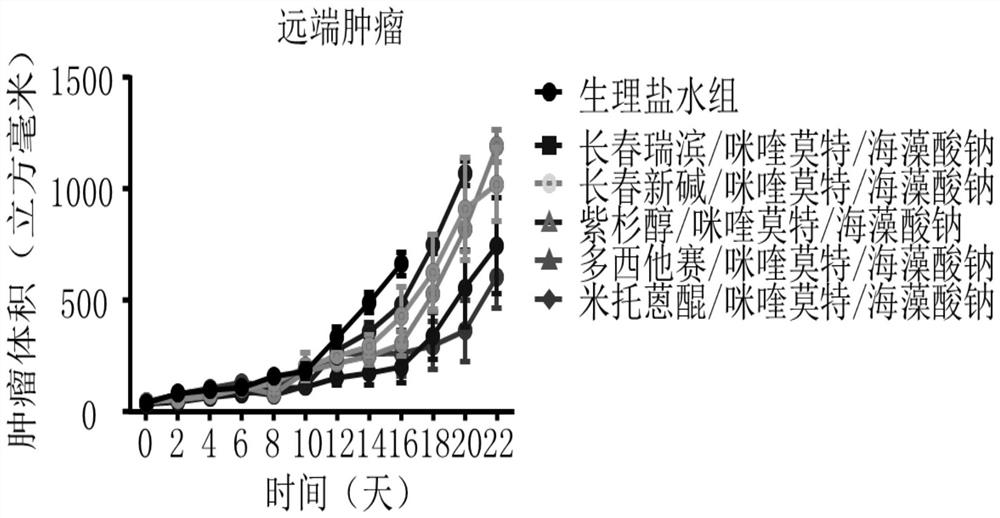
Mitoxantrone composition and preparation method thereof
A technology of mitoxantrone and composition, applied in the field of mitoxantrone composition, can solve problems such as inability to prepare pharmaceutical preparations, dispersion of pharmaceutical components, uncertain dosage, etc., to reduce system exposure level, enhance effect, The effect of reducing toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
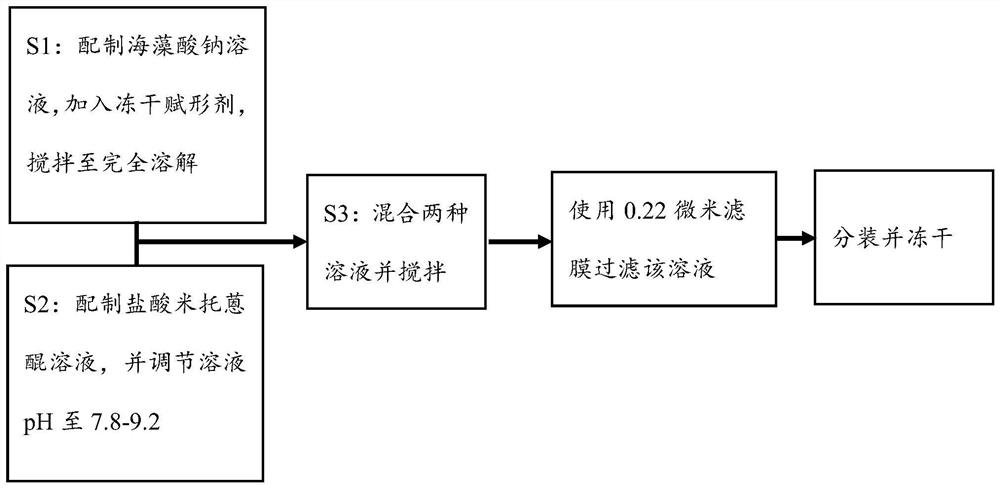
[0039] Example A: Preparation of Formulations
[0040] figure 1 It is a schematic diagram of the preparation and use steps of the mitoxantrone pharmaceutical composition.
Embodiment A1
[0041] Example A1: Preparation of the first composition:
[0042] A certain amount of the immune adjuvant imiquimod R837 solid is weighed and subjected to jet pulverization, and the pulverization pressure is 6-10 bar to obtain micron-scale imiquimod R837. Proportion 1: (0.025~5) Weigh the micron-scale immune adjuvant imiquimod R837 and the surfactant poloxamer 188, preferably 2g R837, add an appropriate amount of poloxamer 188 (0.05g, 0.3g, 0.6g, 1g, 2g, 4g, 6g, 8g, 10g), add 100mL of water for injection, and stir at 100-500rpm for 0.5-2 hours to obtain a suspension. Homogenize the above suspension under high pressure at 750-1200ba for 2-4 times, suck the suspension with a peristaltic pump and fill it into 10mL ampoule bottles, each bottle is 6mL, for a total of 30 bottles. After melting and sealing, a micron suspension is obtained, which is sterilized by moist heat at 105°C to 150°C for 15-20 minutes.
[0043] Poloxamer 188 is a new type of polymer nonionic surfactant. It h...
Embodiment A2
[0065] A certain amount of immune adjuvant Resiquimod (R848) solid is weighed and subjected to jet pulverization, and the pulverization pressure is 6-10 bar to obtain micron-scale Resiquimod (R848).
[0066] Proportion 1: (0.025~5) Weigh the micron-sized immune adjuvant Resiquimod (R848) and the surfactant Poloxamer 407, preferably 0.2g R848, add an appropriate amount of Poloxamer 407 (0.005g, 0.01 g, 0.2 g, 0.4 g, 0.8 g, 1 g), add 200 mL of water for injection, and stir at 100-500 rpm for 0.5-2 hours to obtain a suspension.
[0067] The above suspension is homogenized under high pressure at 750-1200bar for 2-4 times to obtain the suspension, and the suspension is sucked by a peristaltic pump and filled into 10mL ampoule bottles, each bottle of 6mL, for a total of 30 bottles. After melting and sealing, a micron suspension is obtained, which is sterilized by moist heat at 105°C to 150°C for 15-20 minutes.
[0068] Poloxamer 407 is a new type of polymer nonionic surfactant, whi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com