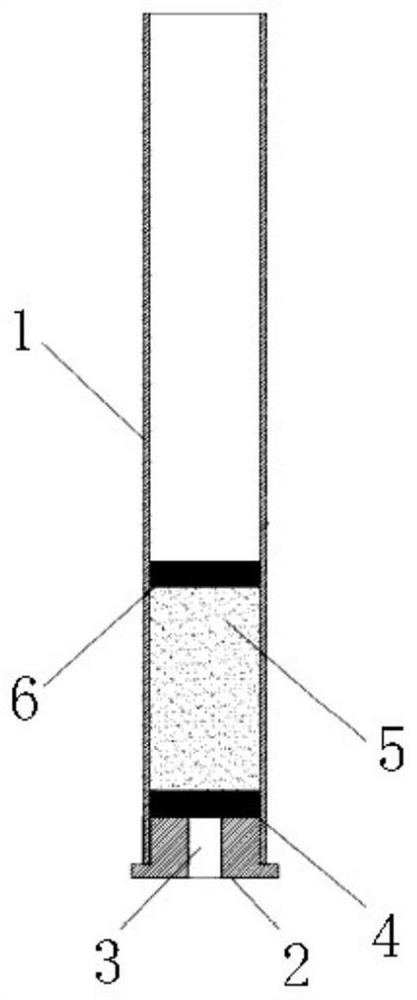
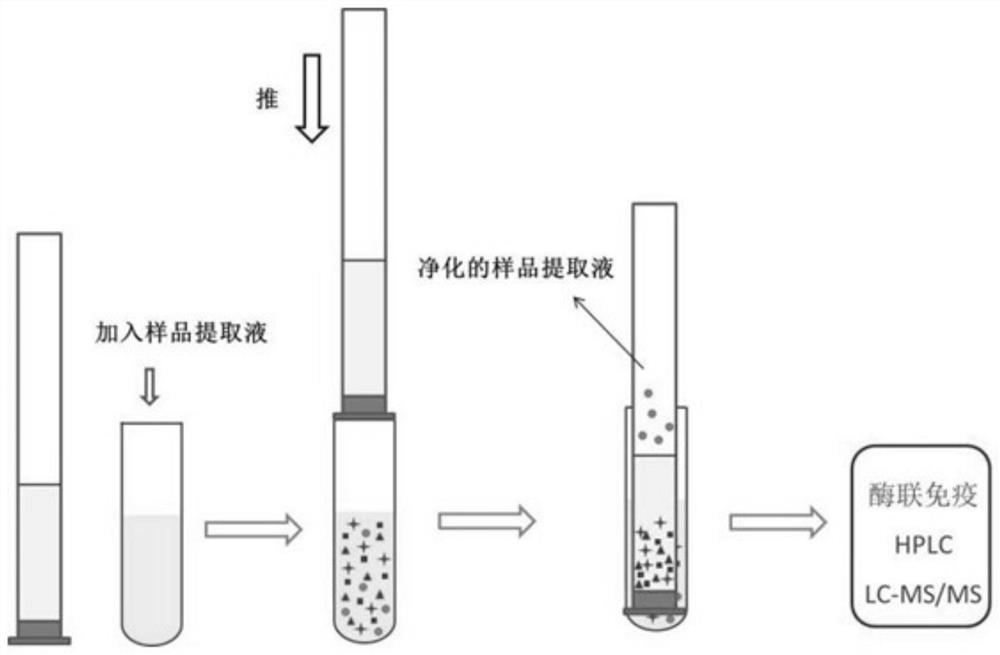
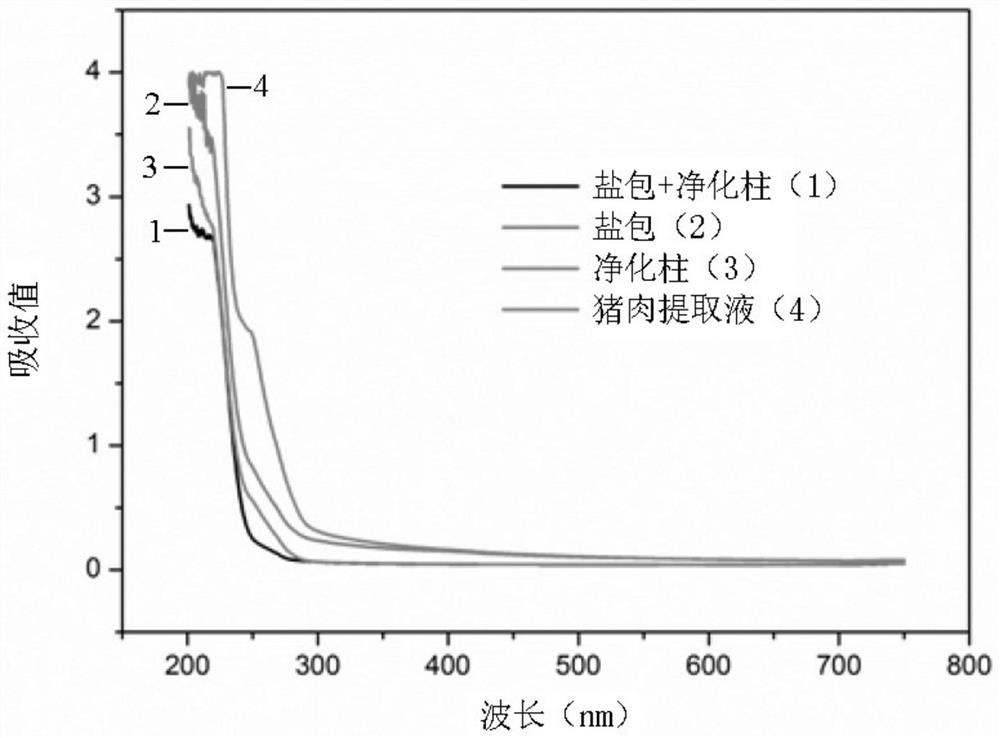
Purifying filler for sulfonamide residues and pretreatment method
A purification column and alumina technology, applied in chemical instruments and methods, separation methods, other chemical processes, etc., can solve the problems of complex operation, high proportion of organic solvents used, high matrix effect, etc., and achieve wide applicable matrix and good stability , High recovery effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1. Verification of the detection effect of sulfonamide drug residues in pork samples
[0069] In Example 1, pork with different concentrations of standard products was used as a sample, and 0.5 g of salt package (sodium sulfate: sodium chloride (mass ratio) = 1:1) and silica gel, alumina and HLB (mass ratio) were used. A solid-phase purification column composed of 1:2:0.5 and a total filler content of 2g). The purified sample extract was diluted 10 times with the initial mobile phase, filtered through a 0.22 μm filter membrane, and finally analyzed by UPLC-MS / MS.
[0070] Test results: under different standard concentrations, the average recoveries obtained were between 70% and 108%, and the RSDs were all less than 15% (see Table 3). It can be seen that this pretreatment method is a qualified sulfonamide drug pretreatment method.
[0071] Table 3. Recovery accuracy and precision of sulfonamide residues in pork samples
[0072]
[0073]
[0074]
Embodiment 2
[0075] Example 2. Verification of the detection effect of sulfonamide drug residues in beef samples
[0076] In Example 2, beef with different concentrations of standard substances was used as the sample, and 1 g of salt package (sodium sulfate: sodium chloride (mass ratio) = 1:0.5) and silica gel, alumina and HLB (mass ratio of 1:3:1, a solid-phase purification column composed of a total filler content of 2g). Take 500 μL of the purified sample extract and blow it dry with nitrogen, reconstitute it with 1 mL of the initial mobile phase, filter it through a 0.22 μm filter membrane, and finally conduct UPLC-MS / MS analysis.
[0077] Test results: under different standard concentrations, the average recovery rate obtained is between 73% and 102%, and the RSD is less than 15% (see Table 4), which is within the scope of the national standard for sulfonamides. It can be seen that this pretreatment method is a qualified sulfonamide drug pretreatment method.
[0078] Table 4. Recove...
Embodiment 3
[0082] Example 3. Verification of the detection effect of sulfonamide drug residues in chicken samples
[0083] In Example 2, chickens with different concentrations of standard products were used as samples, and 0.2 g of salt package (sodium sulfate: sodium chloride (mass ratio) = 1:1) and silica gel, alumina and HLB (mass ratio of 1:0.5:0.5, the total content of filler is 1.5g) of solid phase purification column. Take 200 μL of the purified sample extract and blow dry with nitrogen, reconstitute it with 1 mL of the initial mobile phase, filter it through a 0.22 μm filter membrane, and finally conduct UPLC-MS / MS analysis.
[0084] Test results: under different standard concentrations, the average recovery rate obtained is between 81% and 113%, and the RSD is less than 15% (see Table 5), which is within the scope of the national standard for sulfonamides. It can be seen that this pretreatment method is a qualified sulfonamide drug pretreatment method.
[0085] Table 5. Recove...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com