Chitinase Chi6115 as well as coding gene and application thereof
A technology of chi6115, chitinase, applied in the field of bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
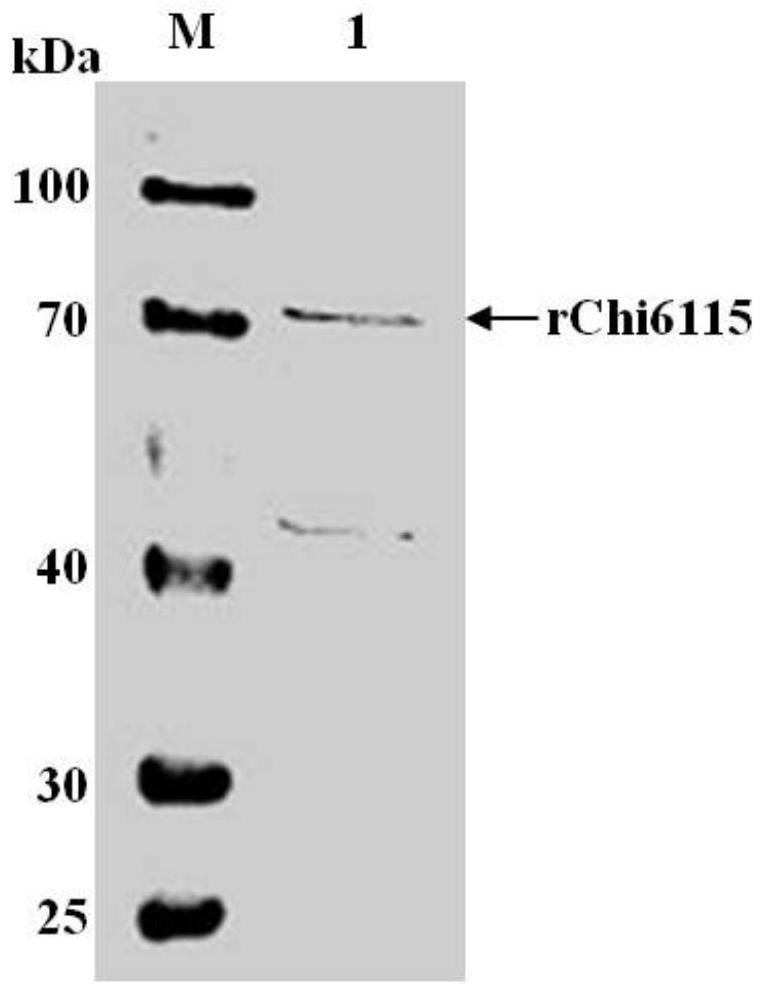
[0028] Example 1 Preparation of chitinase Chi6115
[0029] Proceed as follows:
[0030] Experimental Materials
[0031] Escherichia coli E.coil TOP10 and E.coil BL21 strains (purchased from ThermoFisher), expression vector pET-28a(+) (purchased from Novagen); restriction endonucleases BamHI and XhoI (purchased from Quanjinjin) ; T4 ligase (purchased from Takara); LB medium (1% peptone per liter, 0.5% yeast extract, 1% NaCl); binding buffer (phosphate buffer: 20 mM phosphate, pH 7.4 ); washing buffer (500 mM NaCl, 10-50 mM imidazole, 20 mM phosphate, pH 7.4); elution buffer (500 mM NaCl, 500 mM imidazole, 20 mM phosphate, pH 7.4); 3,5-dinitro Salicylic acid (DNS) (purchased from Shanghai Sangong); Kanamycin (purchased from Shanghai Sangong); Ni SepharoseTM6Fast Flow kit (purchased from Taijing Company); 30kDa ultrafiltration tube (purchased from Taijing Company) ; Chitin monosaccharide and chitobiose standard (purchased from Miragen); chitotriose, chitotetraose, chitopentaos...
Embodiment 2
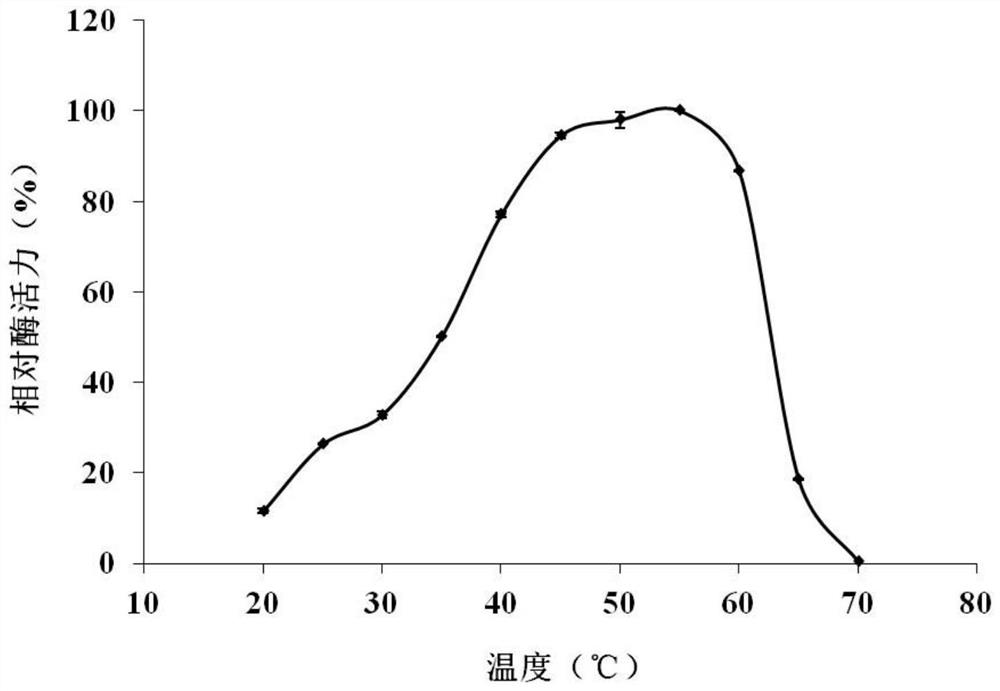
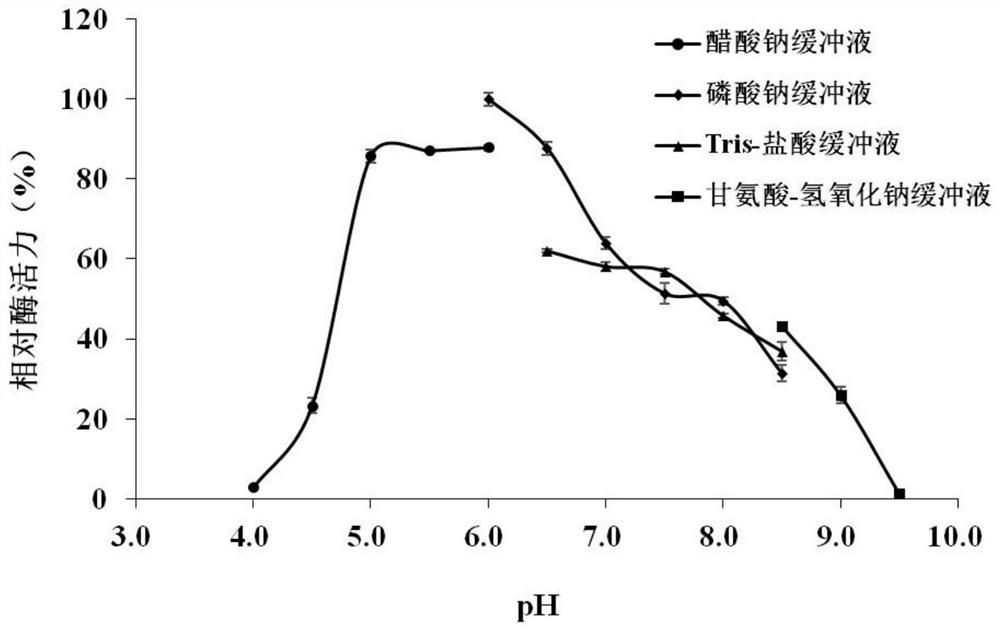
[0042] Enzymatic properties of chitinase Chi6115
[0043] (1) Determination method of chitinase activity
[0044] Preparation of colloidal chitin: Slowly add 5 g of chitin powder to 50 mL of pre-cooled concentrated hydrochloric acid, stir evenly, and let stand at 4°C for 24 hours. Then the mixture was slowly added to 500 mL of pre-cooled deionized water, stirred evenly, and centrifuged at 4° C. and 5000 g for 10 min to collect the chitin precipitate. Repeatedly resuspend the precipitate with deionized water and centrifuge for collection, remove excess hydrochloric acid on the colloidal chitin until the pH value is about 6.5, and store at 4°C for future use.
[0045] The reducing sugar content was determined by DNS method. Mix 25 μL of enzyme solution and 100 μL of 1% colloidal chitin substrate, and react at 55° C. for 2 h. After the reaction was completed, 200 μL of DNS reagent was added, boiled for 5 min, immediately placed in ice water to cool, and the supernatant was tak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com