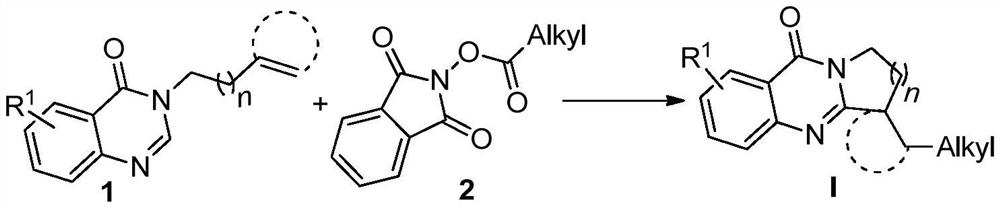
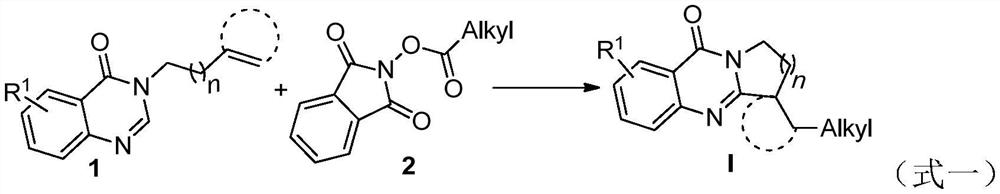
Synthetic method of visible light promoted polycyclic quinazolinone compound initiated by N-hydroxyphthalimide ester decarboxylation
A technology of hydroxyphthalimide esters and polycyclic quinazolinones, which is applied in the field of organic synthesis and can solve problems such as reaction time and reaction condition limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
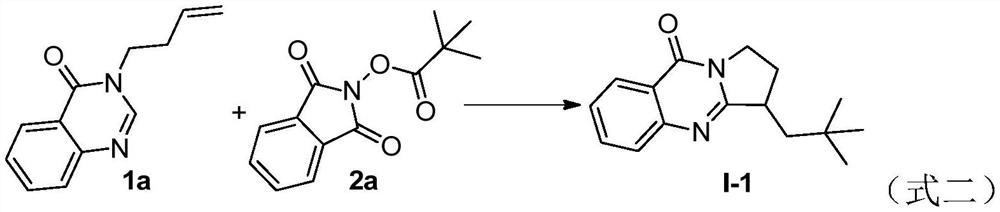
Embodiment 1
[0015]
[0016] The quinazolinone compound represented by formula 1a (40.0 mg, 0.2 mmol), the N-hydroxyphthalimide ester compound represented by formula 2a (14.8 mg, 0.3 mmol), Potassium iodide (KI, 49.8mg, 1.5equiv), triphenylphosphine (PPh 3 , 5.2mg, 10mol%) and DMSO / H 2 O (1.0 mL / 1.0 mL), then the reactor was stirred and reacted under nitrogen atmosphere and 18W blue LED light, and the reaction progress was monitored by TLC until the raw materials disappeared (the reaction time was 24 hours). After the reaction was completed, the reaction solution was reduced to The solvent was concentrated under pressure to remove the solvent, and the residue was separated by column chromatography (elution solvent: ethyl acetate / n-hexane = 5:1) to obtain the target product (72% yield). The structural formula of the product is shown in formula I-1. The data is: 1 H NMR (500MHz, CDCl 3 )δ: 8.28(d, J=8.0, 1H), 7.73-7.69(m, 2H), 7.45-7.42(m, 1H), 4.33-4.29(m, 1H), 3.96-3.91(m, 1H), 3.25...
Embodiment 2
[0018] Sodium iodide was used instead of potassium iodide, and other conditions were the same as in Example 1, and the yield of the target product I-1 was 65%.
Embodiment 3
[0020] The potassium iodide was replaced with lithium iodide, and other conditions were the same as those in Example 1, and the yield of the target product I-1 was 42%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com