Benzene ring fused 5/6/7/6 tetracyclic compound and synthesis method thereof
A tetracyclic compound and synthetic method technology, applied in the field of organic compound synthesis, can solve the problems of low yield and cumbersome reaction steps, and achieve the effects of high yield, single stereoselectivity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
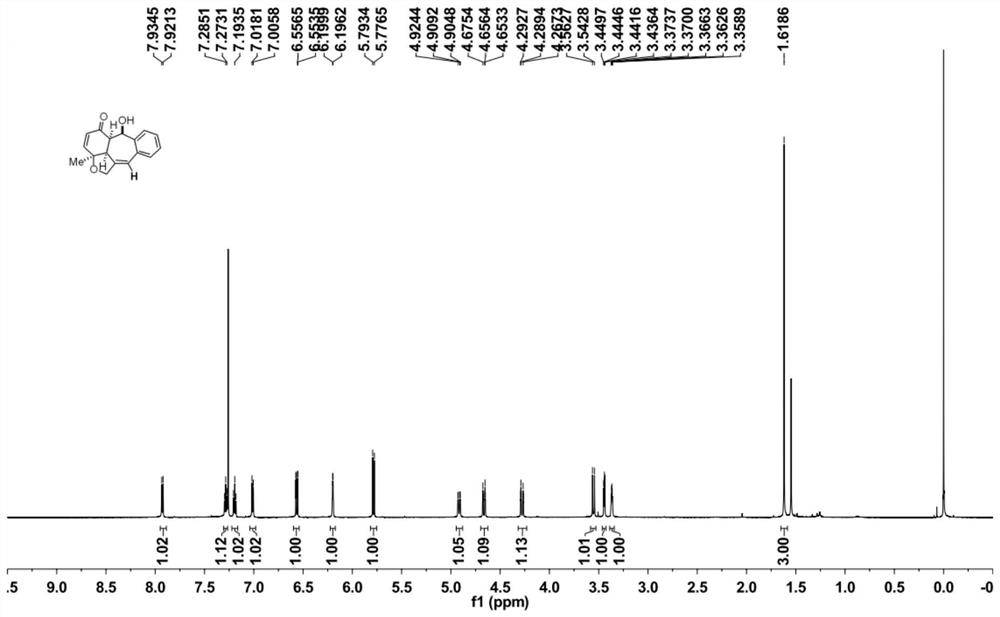
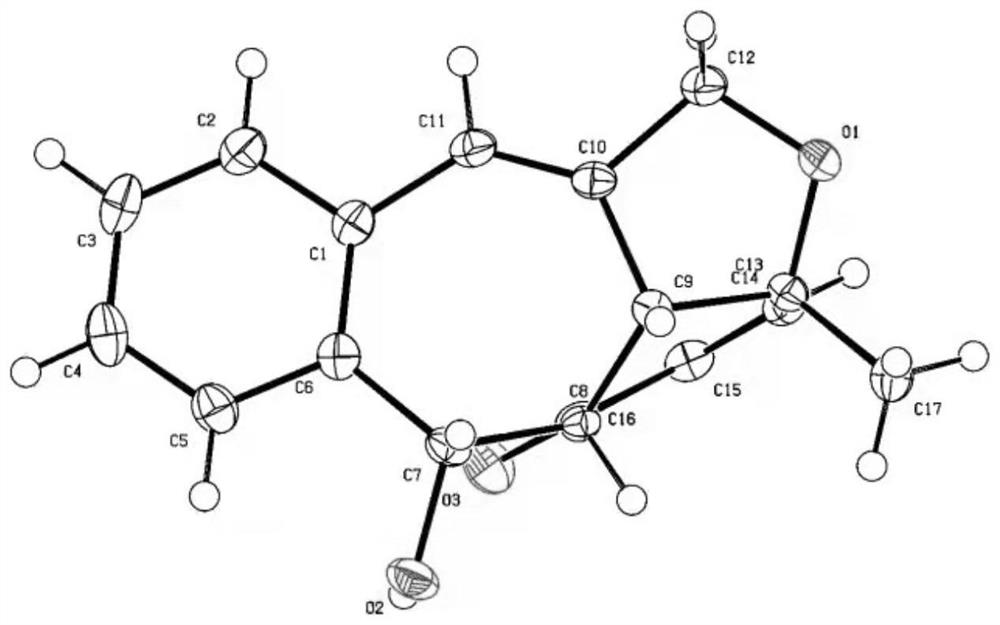
[0034] Take a 10mL round-bottomed flask, add o-alkynylbenzaldehyde 2a (0.2mmol), Molecular sieves (100 mg) and platinum dibromide catalyst (0.01 mmol) were added to tetrahydrofuran (2 mL) under an argon atmosphere, and the reaction was carried out at 70° C. for 12 hours. After cooling to room temperature, tetrabutylammonium tribromide (0.01 mmol) was added to the reaction system, and methanol (2 ml) was further reacted for 1 hour. After the reaction, the insolubles were removed by filtration, water and ethyl acetate were added for extraction twice, the organic phases were combined, dried over anhydrous magnesium sulfate, the filtrate was filtered and concentrated by rotary evaporation, and the crude product was separated by silica gel column chromatography with a volume ratio of 25: The 2-25:8 mixed solution of petroleum ether and ethyl acetate was used as the eluent for gradient elution to obtain 40.2 mg of the target product 1a.
[0035] The reaction equation is:
[0036]...
Embodiment 2
[0044] Take a 10mL round-bottomed flask, add o-alkynylbenzaldehyde 2b (0.2mmol), Molecular sieves (100 mg) and platinum dibromide catalyst (0.01 mmol) were added to tetrahydrofuran (2 mL) under argon atmosphere, and the reaction was carried out at 70° C. for 12 hours. After cooling to room temperature, tetrabutylammonium tribromide (0.01 mmol) was added to the reaction system, and methanol (2 ml) was further reacted for 1 hour. After the reaction, the insolubles were removed by filtration, water and ethyl acetate were added for extraction twice, the organic phases were combined, dried over anhydrous magnesium sulfate, the filtrate was filtered and concentrated by rotary evaporation, and the crude product was separated by silica gel column chromatography with a volume ratio of 25: A mixed solution of petroleum ether and ethyl acetate in a ratio of 1 to 25:6 was used as the eluent for gradient elution to obtain 57.5 mg of the target product 1b.
[0045] The reaction equation i...
Embodiment 3
[0051] Take a 10mL round-bottomed flask, add o-alkynylbenzaldehyde 2c (0.2mmol), Molecular sieves (100 mg) and platinum dibromide catalyst (0.01 mmol) were added to tetrahydrofuran (2 mL) under argon atmosphere, and the reaction was carried out at 70° C. for 12 hours. After cooling to room temperature, tetrabutylammonium tribromide (0.01 mmol) was added to the reaction system, and methanol (2 ml) was further reacted for 1 hour. After the reaction, the insolubles were removed by filtration, water and ethyl acetate were added for extraction twice, the organic phases were combined, dried over anhydrous magnesium sulfate, the filtrate was filtered and concentrated by rotary evaporation, and the crude product was separated by silica gel column chromatography with a volume ratio of 25: A 1-5:1 mixed solution of petroleum ether and ethyl acetate was used as the eluent for gradient elution to obtain 59.7 mg of the target product 1c.
[0052] The reaction equation is:
[0053]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com