Synthesis method of propeller alkane derivative
A derivative, pentane technology, applied in the field of synthesis of chloromethyl propane, can solve the problems of difficult preparation, complicated post-processing, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
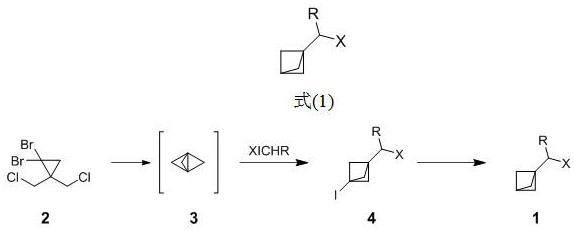
[0022] The present invention provides a method of preparation of a bicyclic [1.1.1] pentane derivative of the preparative formula (1), comprising the following steps:
[0023]
[0024] Step 1): to As the starting material, the formation of tricyclic [1.1.1.0] pentane ([1.1.1]-propellane);
[0025] Step 2): The tricyclic [1.1.1.0] pentane ([1.1.1]-propellal alkane) obtained in step 1) is reacted with the iodine substitute XICHR to form an iodine substitute of the bicyclic [1.1.1] pentane ;
[0026] Step 3): Step 2) result in a bicyclic [1.1.1] pentane iodine , under the action of the initiator, is reduced by the reducing agent ;
[0027] Among them, R is independently selected from hydrogen and C 1-6 Alkyl and halogen, X is independently selected from halogen and C 1-6 Alkyl.
[0028] Preferably, R is independently selected from hydrogen, methyl and Cl, X is independently selected from Cl and methyl; More preferably, R is independently selected from hydrogen, X is independentl...
Embodiment 1
[0050] Example 1: Methyl tert-butyl ether step-by-step method
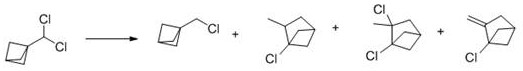
[0051] Preparation of tricyclic [1.1.1.0] pentane (TCP) (Compound 3).
[0052] 5 L dried three-mouth bottle, add raw compound 2 (320 g, 1.09 mol), Ar gas protection, double row needle add CaH 2 Dried methyl tert-butyl ether 1.2 L, stirred until dissolved and clarified, dry ice + isopropanol cooled to internal temperature -45 o C~-15 o C, double row needle dropwise plus methyl lithium solution (800 mL, 2.4 mol, diethoxymethane solution, 3 M), drop-by-drop process greater than 1 h, and then ice water bath reaction for 2 h. The product of methyl tert-butyl ether - diethoxymethane solution was distilled into the receiving bottle, the receiving bottle was cooled with an isopropanol dry ice bath to give 2.5L of methyl tert-butyl ether containing TCP (Compound 3) - diethoxymethane solution.
[0053] Preparation of 1-(chloromethyl)-3-iodine bicyclo [1.1.1]pentane (Compound 5).
[0054] CH was added to a methyl tert-butyl ethe...
Embodiment 2
[0055] Example 2: Ether step-by-step method
[0056] Preparation of tricyclic [1.1.1.0] pentane (TCP) (Compound 3).
[0057] 250 mL dried three-port bottle, add compound raw material 2 (20 g, 67.4 mmol), Ar gas protection, double row needle add dried ether 80 mL, dry ice + isopropanol cooled to internal temperature -45 o C~-15 o C, double row needle dropwise add lithium methyl solution (54 mL, 2.4 mol, diethoxymethane solution, 3 M), the drop dosing process is greater than 1 h, and then the ice water bath reaction is 2 h. The ether-diethoxymethane solution of the product is distilled into the receiving bottle, which is cooled down with an isopropanol dry ice bath. To obtain 120 mL of ether containing TCP (Compound 3) - diethoxymethane solution was obtained.
[0058] Preparation of 1-(chloromethyl)-3-iodine bicyclo [1.1.1]pentane (Compound 5).
[0059] CH is added to the ether-diethoxymethane solution (120 mL) containing TCP (Compound 3). 2 ClI (12.2 g, 67.8 mmol), stirred at room ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com