Triphenylamine-benzopyranium salt derivative NIR-BT-P as well as synthesis method and application thereof
A technology of NIR-BT-P and benzopyrylium salt, which is applied in the field of triphenylamine-benzopyrylium salt derivatives, can solve problems such as measurement difficulties, and achieves low cost, efficient targeting, and simple synthesis. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
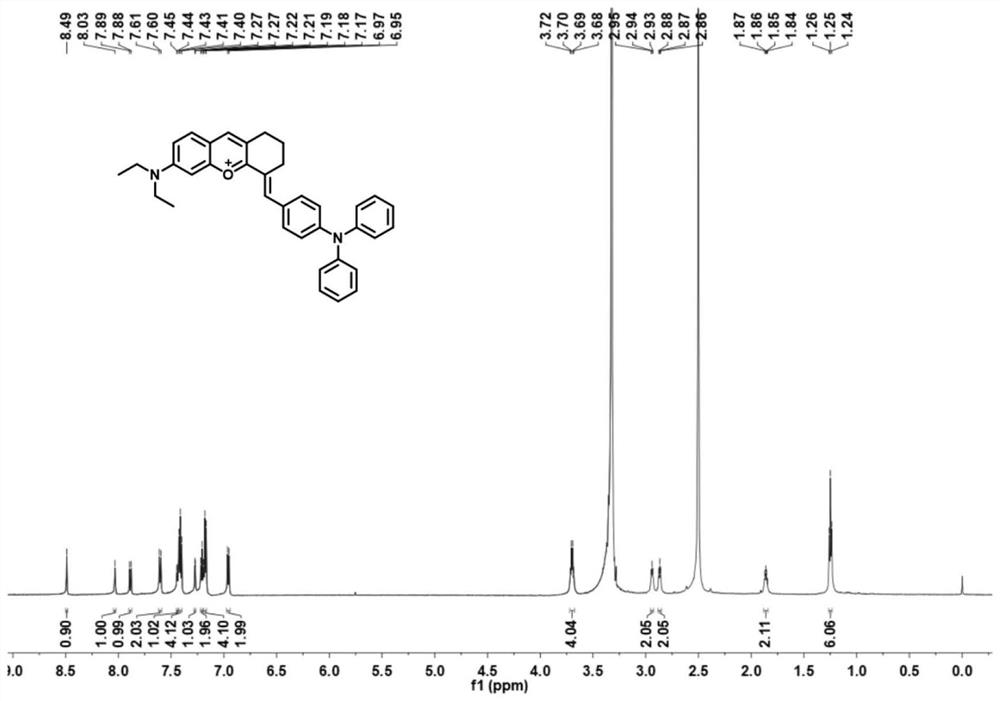
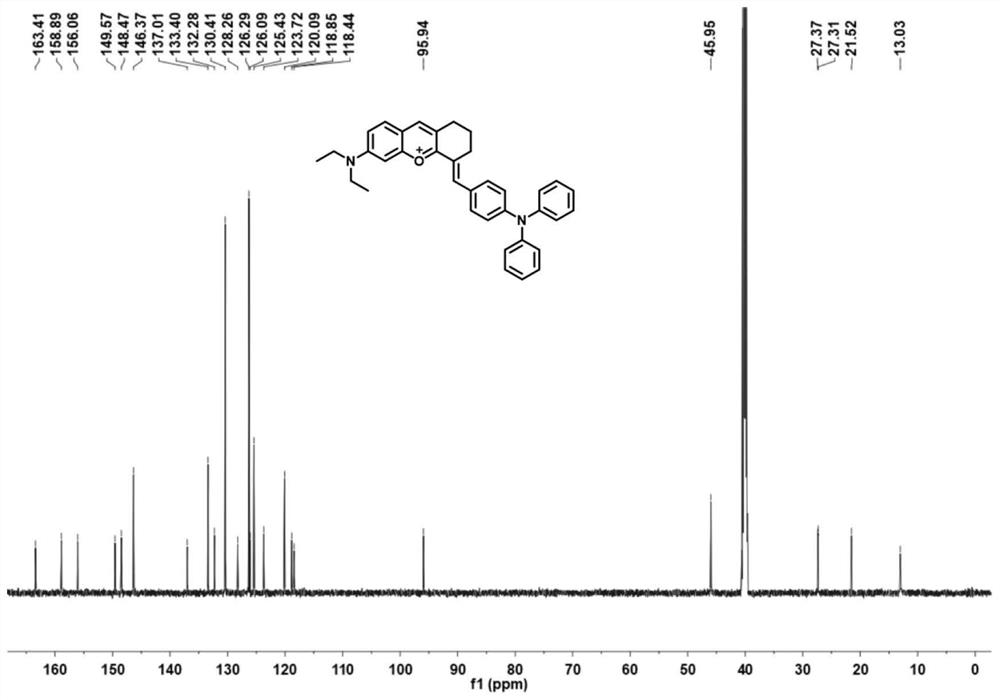
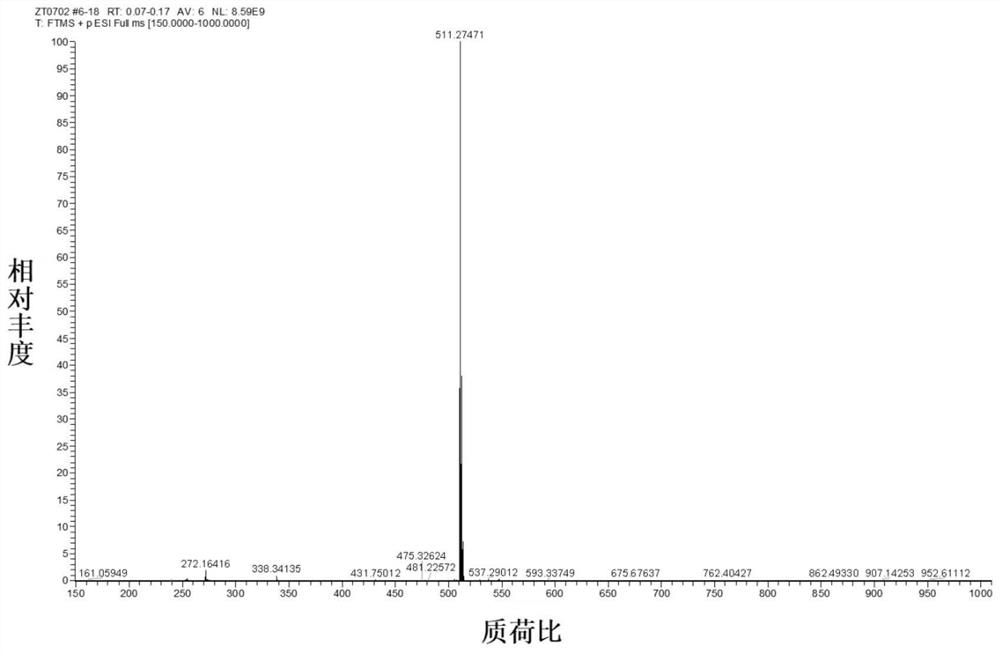
[0035] Preparation and Characterization of NIR-BT-P
[0036] Synthetic route of NIR-BT-P:
[0037]
[0038] The synthetic method of NIR-BT-P, the steps are:
[0039] (1) At 0°C, cyclohexanone (3.1 mL, 30 mmol) was added dropwise to concentrated H 2 SO 4 (40 ml), keeping the solution stirring, then 4-(diethylamino)-2-hydroxybenzaldehyde (2.9 g, 15 mmol) was added. The mixture was further heated at 90°C for 2 hours, and after cooling to room temperature, the mixture was slowly poured into ice water (300 ml), followed by the addition of 4 ml of HClO 4 , the mixture was filtered, washed with water, and dried in vacuo, the obtained brown solid was 6-(diethylamino)-1,2,3,4-tetrahydrooxopyrimidine (5.2 g, yield 97%), which was obtained without further purification. available for the next step.
[0040] (2) 6-(Diethylamino)-1,2,3,4-tetrahydrooxopyrimidine (1.8g, 5mmol) and 4-(diphenylamino)benzaldehyde (2.7g, 10mmol) were dissolved in CH 3 in COOH. The mixture was heated at ...
Embodiment 2
[0042] To prepare a DMSO solution of 2mMNIR-BT-P, add 1,4 dioxane with volumes of 2, 1.9, 1.8, 1.7, 1.6, 1.5, 1.4, 1.3, 1.2, 1.1, 1, 0.9ml to 12 In the fluorescence cuvette, add distilled water in volumes of 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, and 1.1 mL of distilled water to the cuvette, and add 10 μL NIR-BT to it. The DMSO solution of -P was detected on a fluorescence spectrophotometer at the same time. With the increase of the volume ratio of distilled water in the system, the fluorescence intensity at 830 nm gradually decreased. Fluorescence emission map see Figure 4 .
Embodiment 3
[0044] Prepare 2mM NIR-BT-P DMSO solution, prepare 2mM sulfur dioxide aqueous solution; add 1,4 dioxygen with volumes of 1.7, 1.6, 1.5, 1.4, 1.3, 1.2, 1.1, 1, 0.9ml to 9 cuvettes respectively Six rings, and then add distilled water in volumes of 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 1.1 mL, and measure the maximum fluorescence intensity F on a fluorescence spectrometer. max are 556.1, 464.4, 394.4, 314.2, 260.2, 191.9, 142.1, 94.4, 41.1, with the polarity (Δf) as the abscissa, with the fluorescence intensity F max Draw a graph for the ordinate to get the polar working curve; the linear regression equation is: F max =3297.0-10830.5Δf, see Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com