Mutant protein with 27th serine mutation of streptavidin and application of mutant protein
A streptavidin and mutant protein technology, which is applied in the direction of peptides containing affinity tags, applications, peptide preparation methods, etc., can solve the problems of reduced service life, increased use cost, and high use concentration, so as to improve the purification ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、27
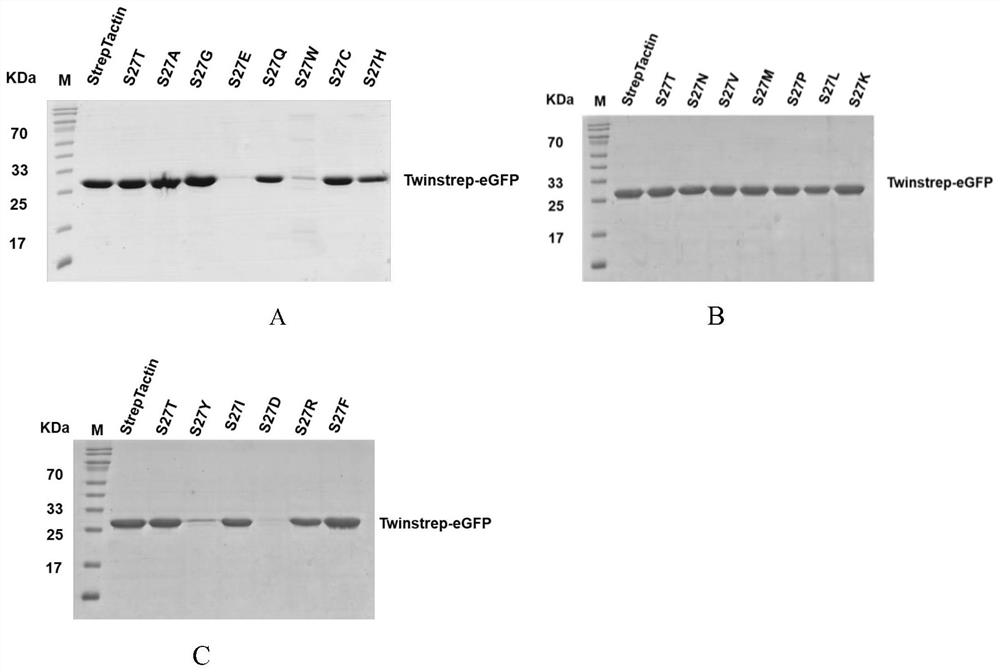
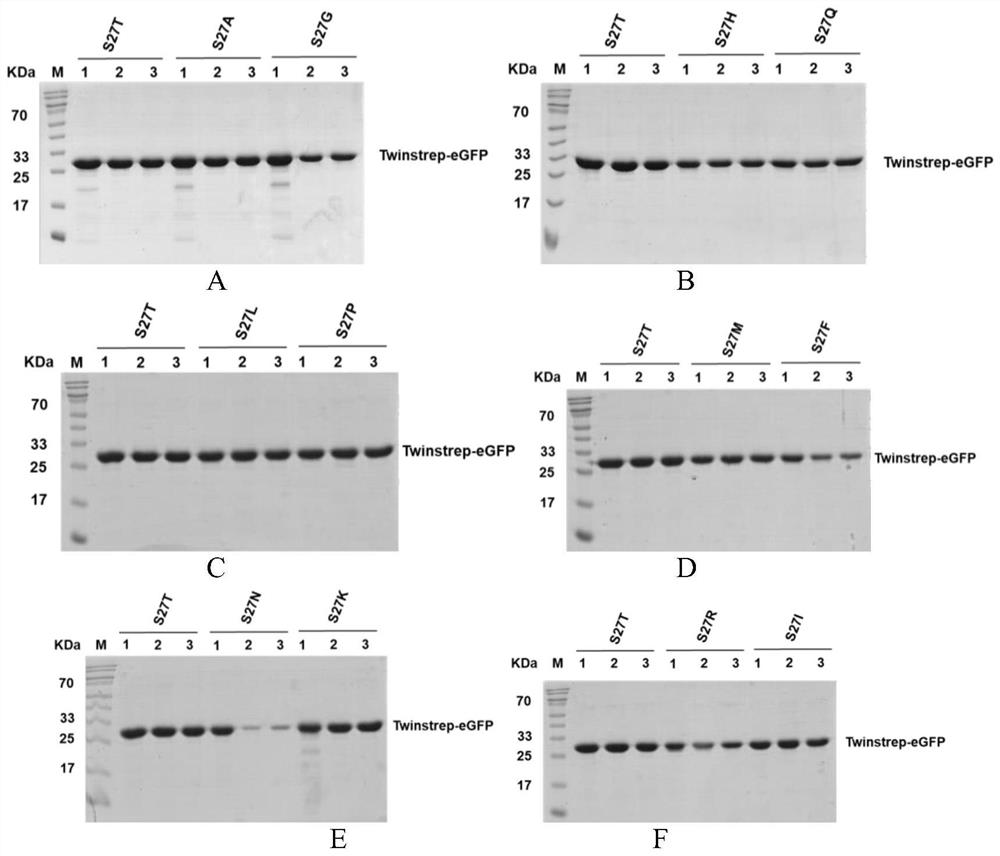
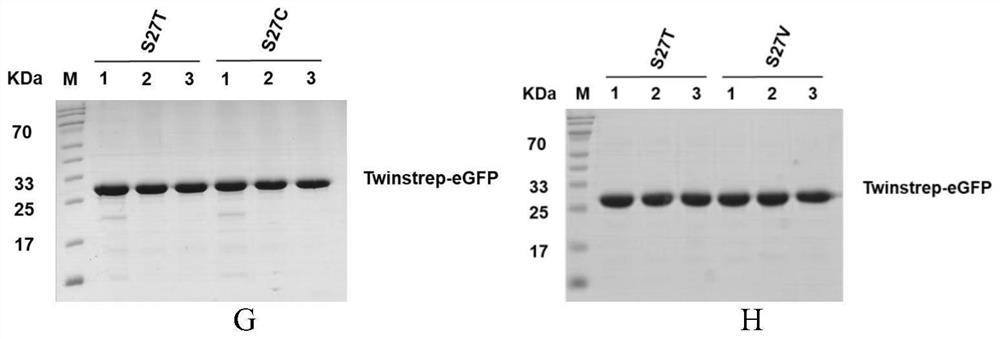
[0024] Example 1, Screening of 27 related mutants enriched for Twinstrep modified proteins
[0025] In the present invention, in order to improve the binding ability of streptavidin (Streptavidin) and Strep tag II, reduce the binding ability of StrepTactin and biotin (Biotin), synthesize the binding site of StrepTactin and its ligand, form hydrogen bond to StrepTactin and biotin Site-directed mutagenesis of the key amino acids, the purpose is to retain the advantages of the original product while making up for its shortcomings, weakening the combination of biotin and StrepTactin, to obtain StrepTactin mutants (StrepTactin muts) that can reversibly bind to biotin. The 27th position was subjected to other amino acid mutations, namely S27T, S27G, S27A, S27C, S27D, S27E, S27F, S27H, S27I, S27K, S27L, S27M, S27N, S27Q, S27R, S27V, S27Y, S27P, S27W, The mutated protein sequences are respectively as SEQ ID NO.4, SEQ ID NO.6, SEQ ID NO.8, SEQ ID NO.10, SEQ ID NO.12, SEQ ID NO.14, SEQ ...
Embodiment 2
[0026] The preparation of embodiment 2, StrepTactin mut
[0027] 1) Take 5 μL of the extracted plasmid containing StrepTactin muts designed above (the plasmid containing StrepTactin mut is based on PIISA-His-StrepTactin as a template, and primers are designed for site-directed mutation. PIISA-His-StrepTactin is synthesized by the company PIISA-His-StrepTactin (as shown in SEQ ID NO.45) was added to 100 μL of BL21 codon plus (DE3) competent cells, ice-bathed for 30 minutes, then heat-shocked at 42°C for 90 seconds, then left on ice for 2 minutes, and 900 μL of LB medium was revived in a shaker at 37°C and 200 rpm for 1 hour, spread on a plate containing 100 μg / mL ampicillin resistance, and incubated overnight at a constant temperature of 37°C;
[0028] 2) On the next day, pick a single colony from the plate cultured overnight and put it into 10 mL LB medium containing 100 μg / mL ampicillin, culture it in a shaker at 37 °C and 200 rpm for 12 hours, and transfer the cultured 10 mL...
Embodiment 3
[0036] Embodiment 3, the cross-linking immobilization of StrepTactin mut
[0037] 1) Take 2mL of the purified His-StrepTactin mut protein, and dissolve the protein in 2L of 200mM NaHCO 3 , 500mMNaCl buffer solution, change the dialysate once every 2 hours, and change the dialysate twice in total;
[0038] 2) The His-StrepTactin mut protein after dialysis is measured for its ultraviolet absorption value at a wavelength of 280nm, and the protein concentration and total protein mass after dialysis are calculated according to the absorption value of 2.84 per mg of protein;
[0039] 3) Cross-link and fix 12 mg of StrepTactin mut protein per milliliter of NHS microspheres (NHS Beads), activate NHS Beads with 1 mM HCl solution 5 times the volume of Beads, and use 200 mM NaHCO with 5 times the volume of Beads after activation 3 1. Equilibrate the NHS Beads with 500mM NaCl buffer, add the dialyzed protein after the equilibrium, and rotate and cross-link at 4°C for 12h;
[0040] 4) Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com