Recombinant staphylococcus aureus for preparing bacterial vesicle multi-combined vaccine as well as preparation method and application of recombinant staphylococcus aureus
A staphylococcus and multiple vaccine technology, applied in the field of biomedicine, can solve the problems of complex preparation procedure, pathogenicity and high cost, and achieve the effects of improving preparation efficiency, simplifying purification process and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 Knockout and identification of agrA gene in Staphylococcus aureus
[0077]S. aureus is the first Gram-positive bacterium that has been confirmed to produce MVs (Lee et al., Proteomics, 2009; 9:5425–36), but the MVs produced by wild-type S. aureus are virulent and affect MVs Safety of use (Yuan et al., Nano Lett, 2018; 18:725-33), this application knocks out the agrA gene of the quorum sensing system in Staphylococcus aureus RN4220 to construct a safe strain of Staphylococcus aureus;
[0078] 1. Construction of knockout vector
[0079] The agrA gene sequence on the genome of Staphylococcus aureus RN4220 strain was analyzed as SEQ ID NO: 6, and the amino acid sequence of the AgrA protein was SEQ ID NO: 1. Design primers at about 900bp upstream and downstream of the knockout sequence site. For the left homology arm amplification primers, see the sequence listing SEQ ID NO: 19 (primer P1) and 20 (primer P2), and for the right homology arm amplification primers,...
Embodiment 2
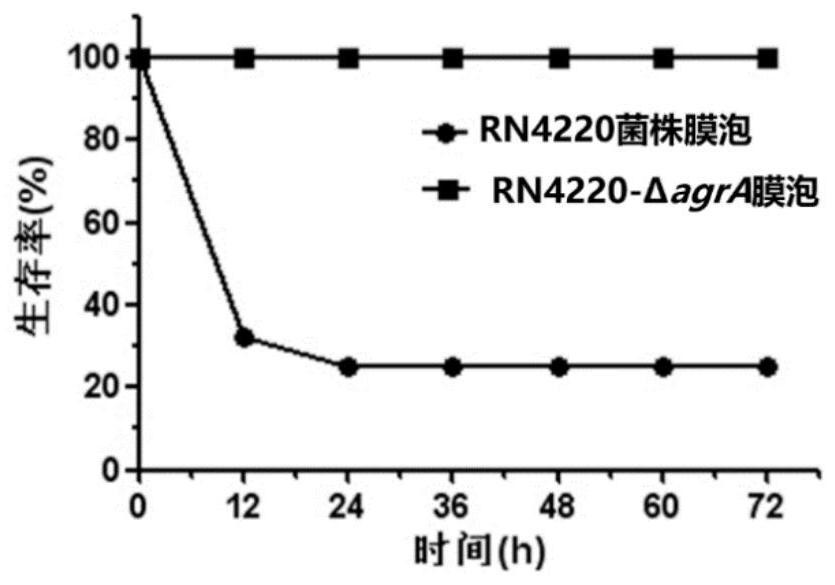
[0095] Example 2 Virulence Detection of Membrane Vesicles of Staphylococcus aureus RN4220-ΔagrA Knockout Strain
[0096] The quorum sensing system Agr is an important virulence regulation system of S. aureus, which controls the expression of hundreds of virulence factors. The loss of this system function will lead to a significant decrease in the virulence of the strain (Reye et al., JBacteriol, 2011; 193: 6020-31); In order to observe the safety performance of bacterial membrane vesicles after agrA knockout, the applicant prepared membrane vesicles of RN4220-ΔagrA knockout strain and its wild strain, and used Balb / c mouse animal experiment to detect the toxicity of membrane vesicles. To verify the safety of membrane vesicles of RN4220-ΔagrA knockout strain.
[0097] 1. Cultivation of Staphylococcus aureus RN4220 and RN4220-ΔagrA
[0098] Pick a single colony from the BHI solid plate, inoculate it in 3ml of BHI liquid medium, culture it with shaking at 37°C for 18 hours, in...
Embodiment 3
[0105] Example 3 Construction of Staphylococcus aureus RN4220-ΔagrA / lcrV engineering bacteria
[0106] It has been reported that Eno of Staphylococcus aureus can be presented in membrane vesicles (Yuan et al., Nano Lett, 2018; 18:725-33). This application uses enolase Eno (48kDa) as the fusion target molecule in Staphylococcus aureus, The fusion protein of Yersinia pestis protective antigen LcrV and Eno was constructed by genetic engineering technology, and the fusion protein was presented in MVs by using the membrane vesicle localization function of Eno.
[0107] 1. Selection of LcrV molecules
[0108] Yersinia pestis (Yersinia pestis) is a bacillus in the genus Yersinia. It is the pathogen of bubonic plague, pneumonic plague and septicemic plague. Deadly disease; the main clinical manifestations are high fever, swollen and painful lymph nodes, bleeding tendency, special inflammation of the lungs, etc.; it has been recorded as far back as more than 2,000 years ago, and three...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com