Cyano-containing polysubstituted aromatic diamine monomer and preparation method thereof
An aromatic diamine and monomer technology, applied in the field of diamine monomer and its preparation, can solve the problems of poor melt processability and solubility of polyimide, limited popularization and application, corrosion of mechanical equipment and the like, and achieves good solubility, The effect of low temperature and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] (1) Under the protection of nitrogen, in a three-necked flask equipped with mechanical stirring, add a molar ratio of 2:1 containing substituent R 1 The p-aminophenol A and dihalobenzonitrile compound B were further separated into ionic liquid catalysts, stirred at room temperature for half an hour, then heated to 65°C for 10 hours, and then ended the reaction;
[0065] (2) White diamine monomer can be obtained after settling, filtering, drying and recrystallization;
[0066] In above-mentioned reaction, described ionic liquid catalyst selects ionic liquid catalyst 1, and the structural formula of described ionic catalyst is: The dosage is containing substituent R 1 2 times the mass sum of p-aminophenol A and dihalobenzonitrile compound B, Ar 1 Choose as: Ar 2 Choose as: The chemical structural formula of gained target product is:
[0067]
[0068] The specific characterization data are as follows:
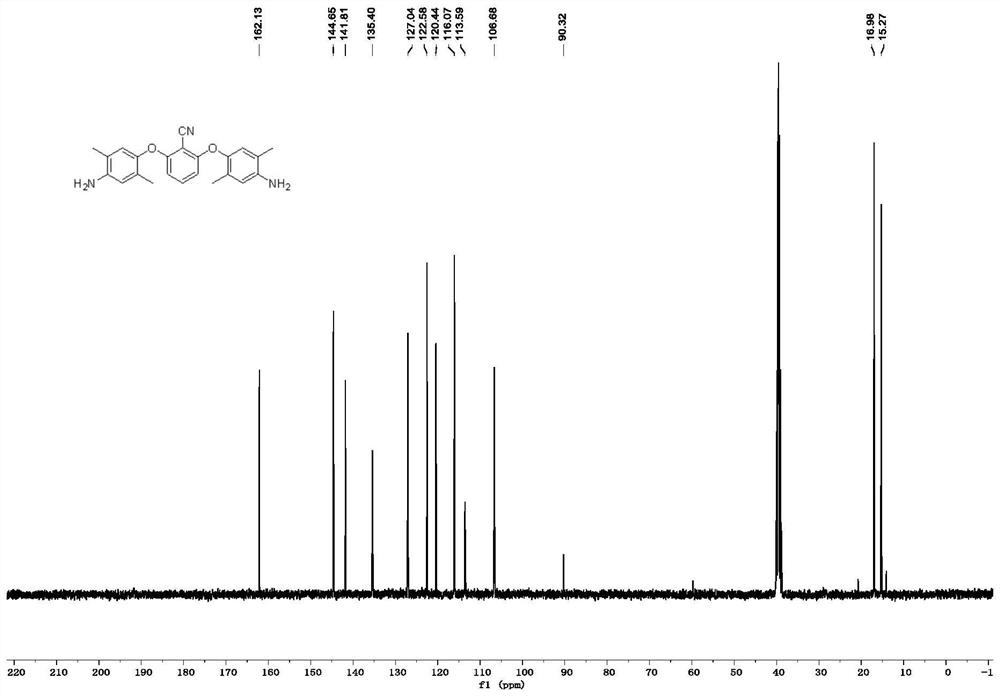
[0069] like figure 1 as shown, 13 C NMR (101MHz, DMSO-d...
Embodiment 2-7
[0073] The specific method is consistent with that of Example 1, except that the reaction temperature, reaction time, and molar ratio of raw materials will be somewhat different. The specific setting parameters and the purity of the target product are shown in Table 1.
Embodiment 8
[0075] Concrete method is consistent with embodiment 1, and difference is as shown in table 1, and ionic liquid catalyst selects ionic liquid catalyst 4 for use, and the structural formula of described ionic catalyst 4 is: The dosage is containing substituent R 1 2 times of the mass sum of p-aminophenol A and dihalobenzonitrile compound B, the chemical structural formula of the obtained target product is:
[0076]
[0077] The specific characterization data are as follows:
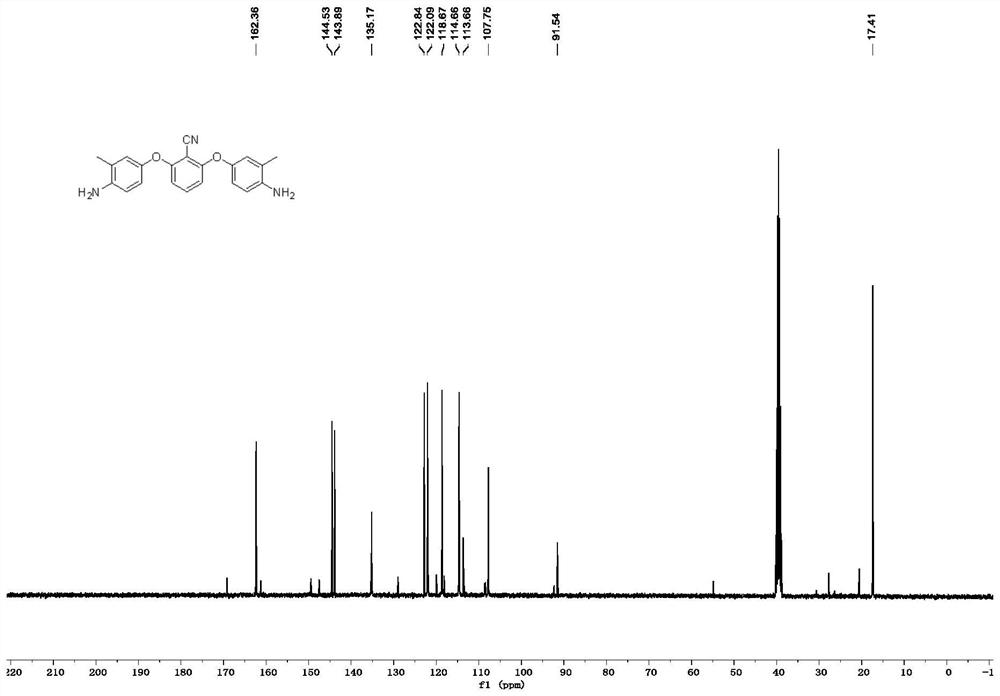
[0078] like image 3 as shown, 13 C NMR (101MHz, DMSO-d 6 )δ162.4, 144.5, 143.9, 135.2, 122.8, 122.1, 118.7, 114.7, 113.7, 107.8, 91.5, 17.4. The carbon spectrum is consistent with the structure of the expected product spectrum.
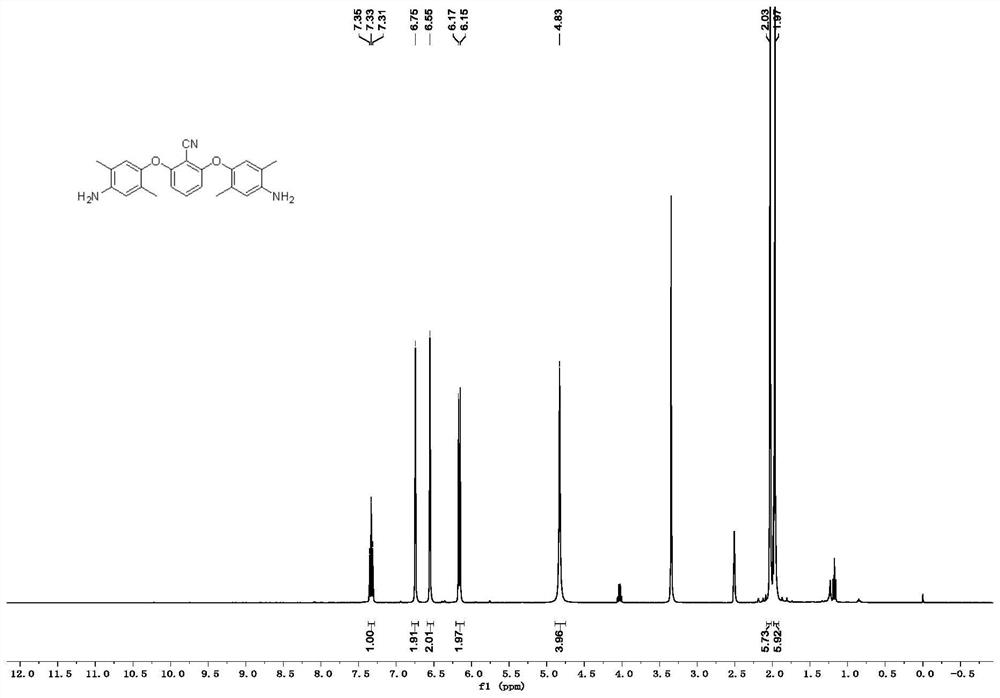
[0079] like Figure 4 as shown, 1 HNMR (400MHz, DMSO-d 6 )δ7.36(t, J=8.5Hz, 1H), 6.83–6.81(m, 2H), 6.78–6.75(m, 2H), 6.69–6.67(m, 2H), 6.31(d, J=8.5Hz ,2H), 4.89(s,4H). The hydrogen spectrum is consistent with the structure of the expected product.
[0080] The speci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com