Application and preparation of diketopiperazine natural product and derivative and obtained derivative
A technology of diketopiperazines and natural products, applied in the fields of drug combination, organic chemistry, antitumor drugs, etc., can solve the problems of low natural content, restricted application and development, lack of derivative development and application, etc. Good effect on tumor activity and anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
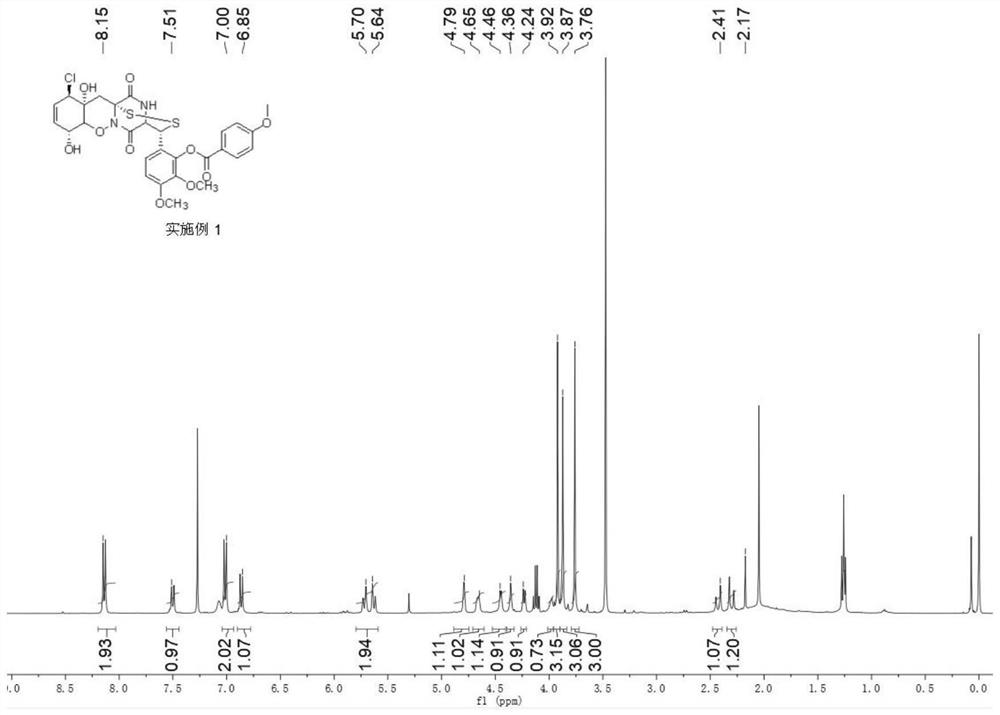
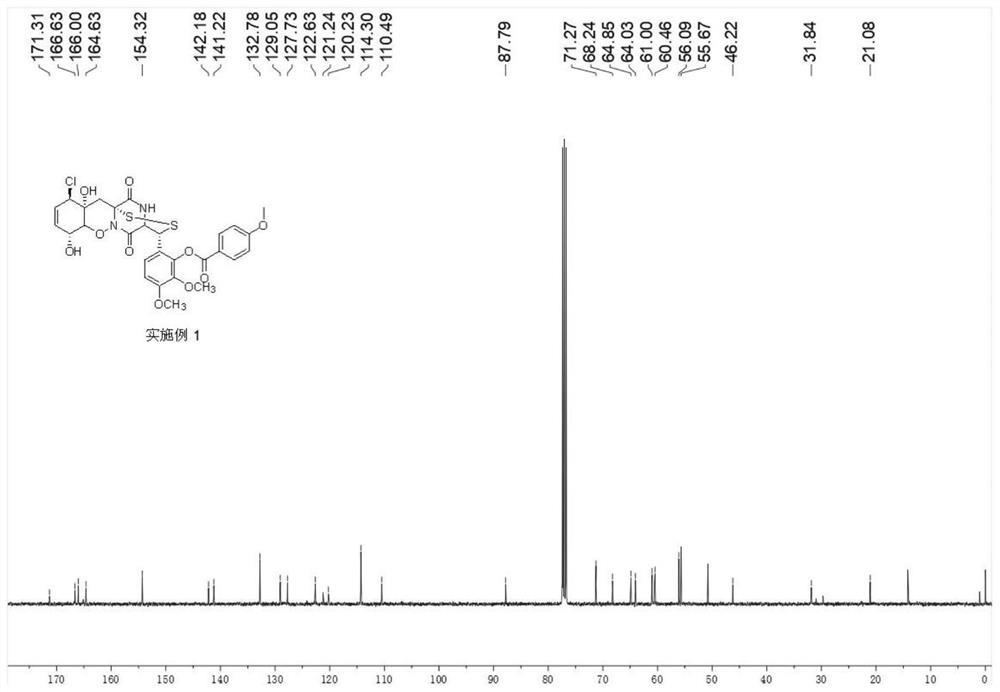
[0058] Synthesis of 6-((3R,8R,11R,11aR,12aR)-11-chloro-8,11a-dihydroxy-5,13-dioxy-4,5,7a,8,11a,12 having the following structural formula -Hexahydro-3H, 11H-4,12a-(epiaminomethyl)benzo[5,6][1,2]oxazine[3,2-c][1,2,4]dithia 3- base)-2,3-dimethoxyphenyl 4-methoxybenzoate:
[0059]
[0060] Its preparation process is:
[0061] Under cooling in an ice bath, 27 μL of 4-methoxybenzoyl chloride (0.2 mmol) and 28 μL of triethylamine (0.2 mmol) were sequentially added to the acetone solution of 20 mg (0.04 mmol) Pretrichodermamide B, and the reaction was stirred at room temperature for 4 h. The liquid was concentrated under reduced pressure to obtain a crude yellow oily product, which was separated by VLC method (gradient elution with ethyl acetate and petroleum ether) to obtain 24 mg of a white solid product with a yield of 92.3%.
[0062] The reaction formula to obtain the product is as follows:
[0063]
[0064] The product was characterized by proton nuclear magnetic spectr...
Embodiment 2
[0071] Synthesis of 6-((3R,8R,11R,11aR,12aR)-11-chloro-8,11a-dihydroxy-5,13-dioxy-4,5,7a,8,11a,12 having the following structural formula -Hexahydro-3H, 11H-4,12a-(epiaminomethyl)benzo[5,6][1,2]oxazine[3,2-c][1,2,4]dithia 3- base)-2,3-dimethoxyphenyl 4-(trifluoromethyl)benzenesulfonate:
[0072]
[0073] Its preparation process is:
[0074] Use 4-trifluoromethylbenzenesulfonyl chloride to replace the 4-methoxybenzoyl chloride in Example 1, and the rest of the preparation process is the same as in Example 1, separated by VLC (elution with ethyl acetate and petroleum ether gradient), In the elution gradient of petroleum ether: ethyl acetate = 1:1, 0.9 mg of white solid product was obtained (yield 3.2%).
[0075] The reaction formula to obtain the product is as follows:
[0076]
[0077] The product was characterized by proton nuclear magnetic spectrum, carbon spectrum, electrospray ion mass spectrometry and electrospray high-resolution mass spectrometry. The results are...
Embodiment 3
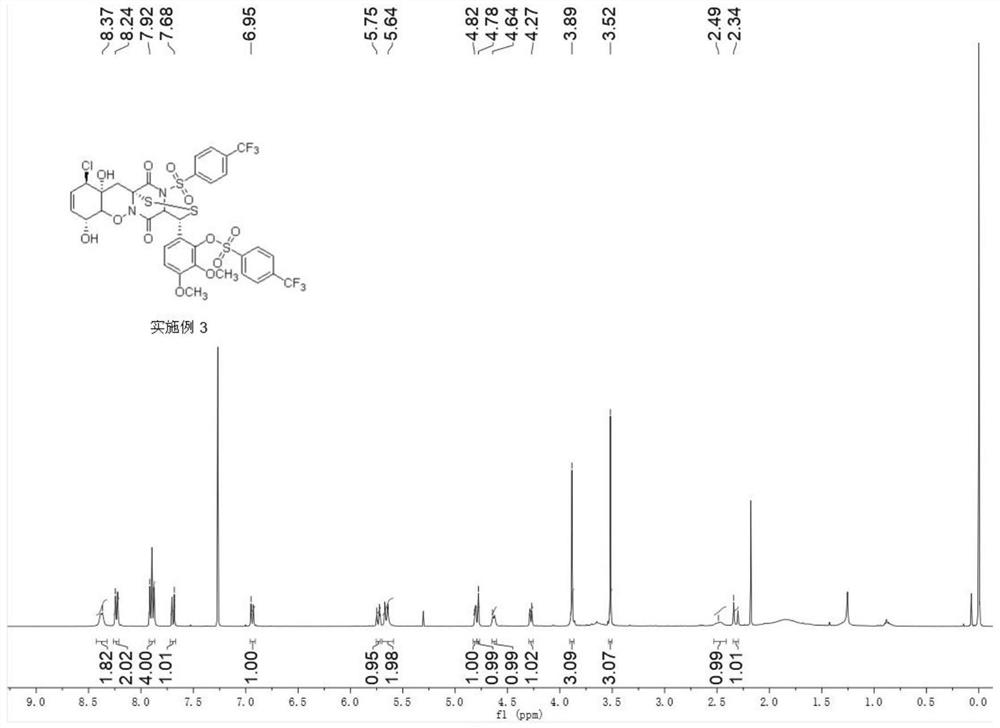
[0083] During the preparation of Example 2, 6-((3R, 8R, 11R, 11aR, 12aR)-11-chloro-8,11a-dihydroxy-5,13- Dioxy-14-((4-(trifluoromethyl)phenyl)sulfonyl)-4,5,7a,8,11a,12-hexahydro-3H,11H-4,12a-(epiaminomethane ) benzo[5,6][1,2]oxazine[3,2-c][1,2,4]dithian 3-yl)-2,3-dimethoxyphenyl 4-(three Fluoromethyl)benzenesulfonate:
[0084]
[0085] Separation by VLC method (gradient elution with ethyl acetate and petroleum ether), 14.5 mg of the white solid product can be obtained at the elution gradient of petroleum ether: ethyl acetate = 2:1, with a yield of 40.1%.
[0086] The product was characterized by proton nuclear magnetic spectrum, carbon spectrum, electrospray ion mass spectrometry and electrospray high-resolution mass spectrometry. The results are as follows:
[0087] 1 HNMR (400MHz, CDCl 3 )δ8.38(d, J=8.1Hz, 2H), 8.23(d, J=8.3Hz, 2H), 7.89(t, J=8.3Hz, 4H), 7.69(d, J=8.9Hz, 1H) ,6.94(d,J=9.0Hz,1H),5.74(dt,J=10.5,2.5Hz,1H),5.66(dt,J=10.5,2.1Hz,2H),4.83–4.80(m,1H), 4.78(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com