Preparation method of defect-rich Mn-Co metal oxide catalyst
An oxide, defect-rich technology, used in metal/metal oxide/metal hydroxide catalysts, catalyst activation/preparation, physical/chemical process catalysts, etc., can solve problems such as unfavorable active site exposure, hard agglomeration, etc. Achieve the effect of inhibiting metal ion agglomeration and refining metal ion dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
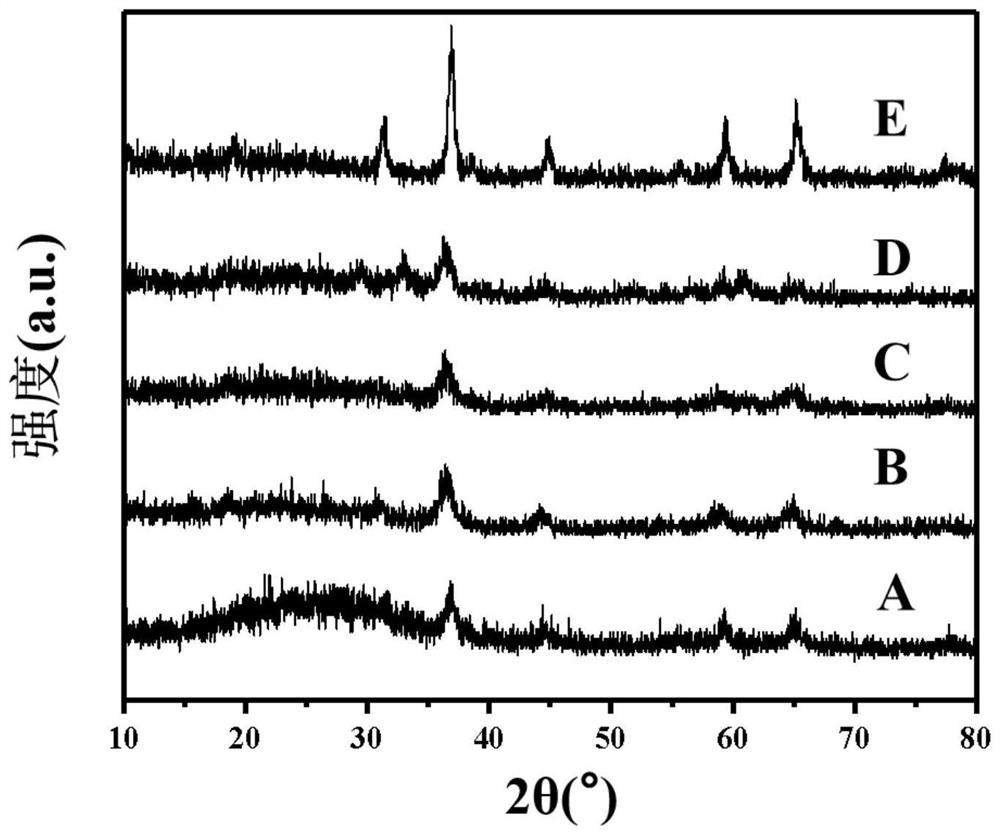
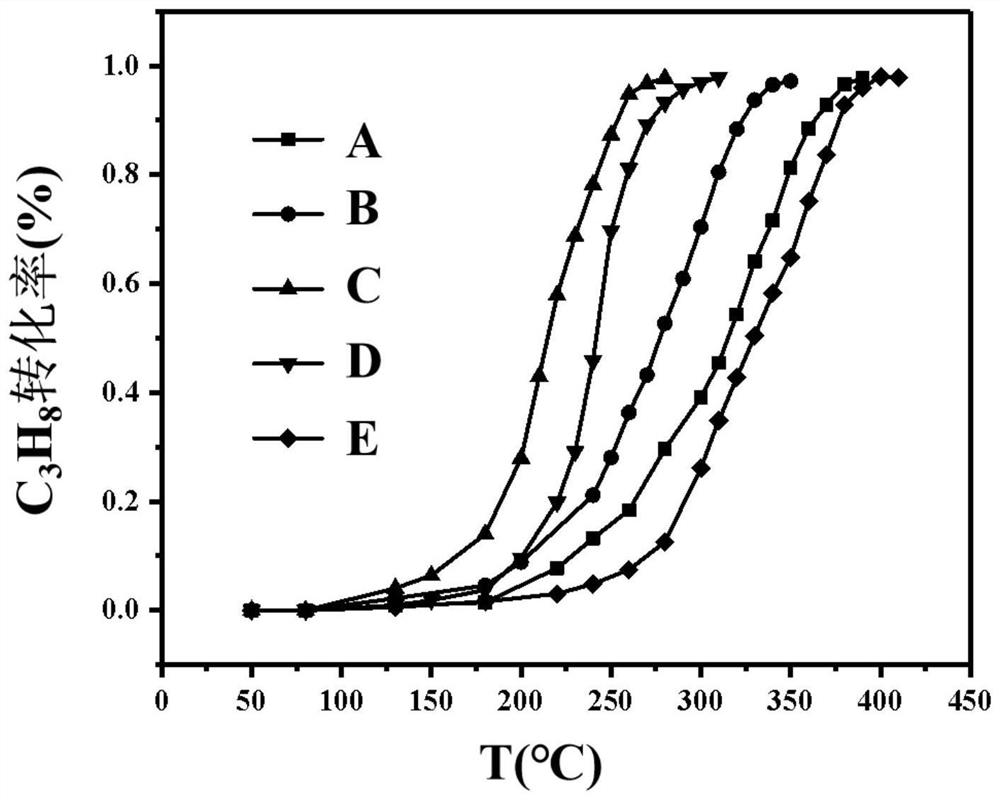
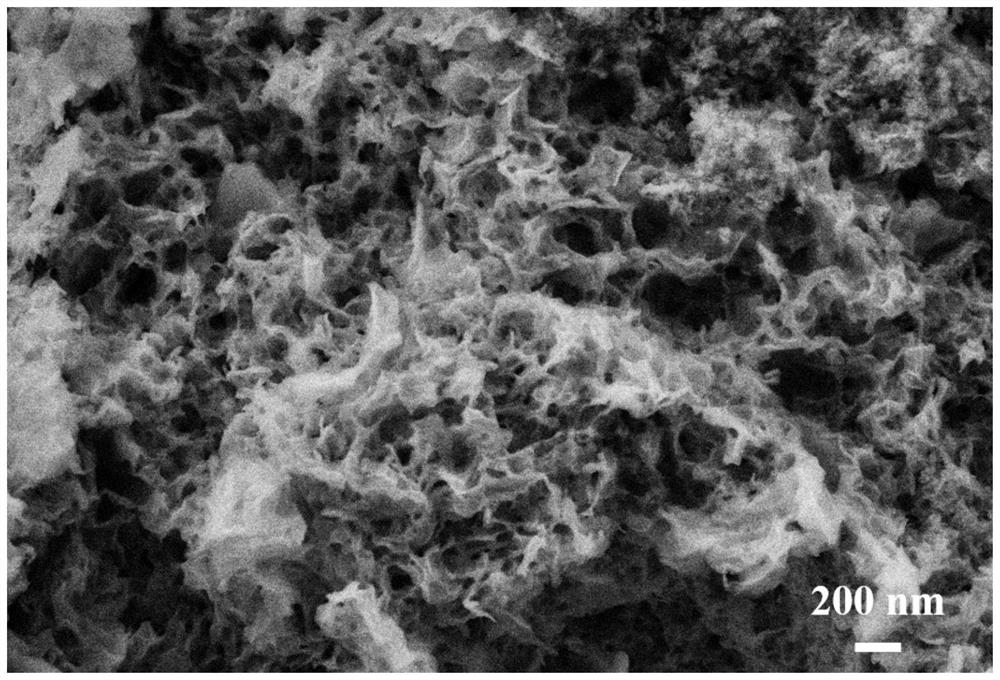
[0021] Take 0.1g Mn(NO 3 ) 2 4H 2 O, 0.47g Co(NO 3 ) 2 ·6H 2 O, 0.420g of 1,3,5-benzenetricarboxylic acid was mixed and placed in a ball mill jar. Dissolve 160 μL of formic acid in 1 mL of N,N-dimethylformamide, then transfer the solution to a ball mill, mix it with solid powder to obtain a suspension, add 420 μL of triethylamine for deprotonation treatment. Tighten the ball mill jar, and fix it in the slot of the vibratory high-energy ball mill, and the milling reaction time is 30 minutes. Centrifuge the product with N,N-dimethylformamide and absolute ethanol and filter, and dry the remaining solid powder in a drying oven at 60°C for 2 hours, and calcinate in a muffle furnace to obtain the target Mn-Co metal oxide catalyst (A), the sample BET specific surface area is 62.8m 2 g -1 . The calcination atmosphere is air, the calcination temperature is 400°C, the heating rate of the muffle furnace is 4°C / min, and the holding time is 2 hours.
Embodiment 2
[0023] Take 0.2g Mn(NO 3 ) 2 4H 2 O, 0.35g Co(NO 3 ) 2 ·6H 2 O, 0.420g of 1,3,5-benzenetricarboxylic acid was mixed and placed in a ball mill jar. Dissolve 160 μL of formic acid in 1 mL of N,N-dimethylformamide, then transfer the solution to a ball mill, mix it with solid powder to obtain a suspension, add 420 μL of triethylamine for deprotonation treatment. Tighten the ball mill jar, and fix it in the slot of the vibratory high-energy ball mill, and the milling reaction time is 30 minutes. The product was centrifuged with N,N-dimethylformamide and absolute ethanol and filtered, and the remaining solid powder was dried in a drying oven at 60°C for 2 hours, and calcined in a muffle furnace to obtain the target Mn-Co metal oxide catalyst (B), the sample BET specific surface area is 72.3m 2 g -1 . The calcination atmosphere is air, the calcination temperature is 400°C, the heating rate of the muffle furnace is 4°C / min, and the holding time is 2 hours.
Embodiment 3
[0025] Take 0.3g Mn(NO 3 ) 2 4H 2 O, 0.23g Co(NO 3 ) 2 ·6H 2 O, 0.420g of 1,3,5-benzenetricarboxylic acid was mixed and placed in a ball mill jar. Dissolve 160 μL of formic acid in 1 mL of N,N-dimethylformamide, then transfer the solution to a ball mill, mix it with solid powder to obtain a suspension, add 420 μL of triethylamine for deprotonation treatment. Tighten the ball mill jar, and fix it in the slot of the vibratory high-energy ball mill, and the milling reaction time is 30 minutes. The product was centrifuged with N,N-dimethylformamide and absolute ethanol and filtered, and the remaining solid powder was dried in a drying oven at 60°C for 2 hours, and calcined in a muffle furnace to obtain the target Mn-Co metal oxide catalyst (C), the sample BET specific surface area is 85.8m 2 g -1 . The calcination atmosphere is air, the calcination temperature is 400°C, the heating rate of the muffle furnace is 4°C / min, and the holding time is 2 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com