Combination
A composition and analog technology, applied in the field of combination, can solve the problem of no medicine for Huntington's disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
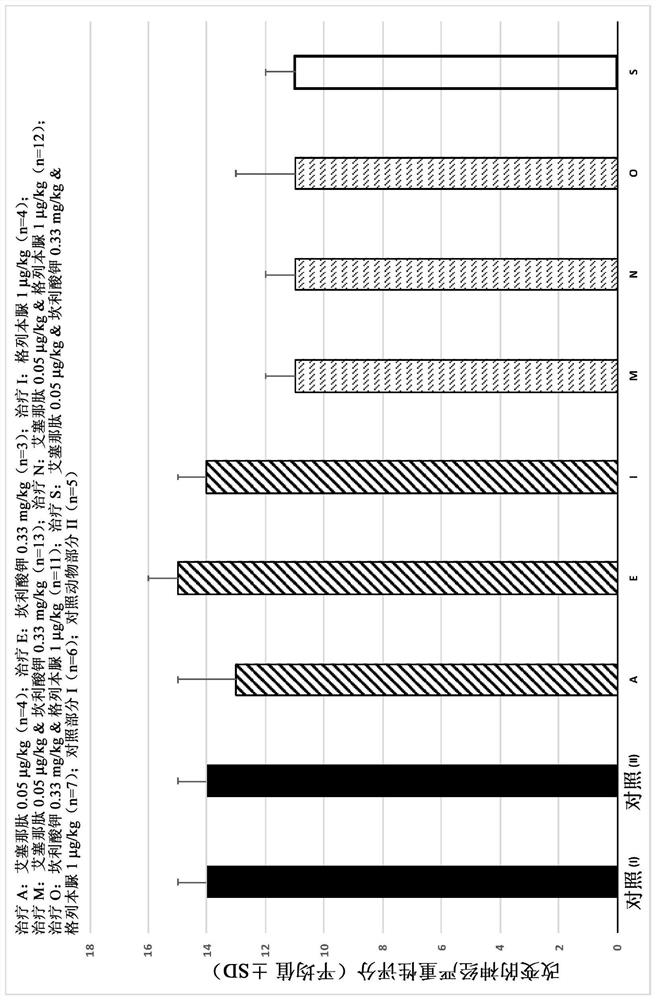
[0623] Example 1. Dose Effect Study of the Efficacy of Exenatide, Canrenoate Potassium and Glibenclamide and Their Combinations in the Rat Model of Cerebral Ischemia and Reperfusion Injury
[0624] The aim of this study was to evaluate (a) the neuroprotection of glibenclamide, exenatide and canrenoate potassium in a rat model of cerebral ischemia and reperfusion injury after repeated intravenous administration as monotherapy Dose Effect of Effects (Study Part I) and Effect of (b) Compound Combination Compared to the Corresponding Monotherapy (Study Part II).
[0625] Transient middle artery occlusion (t-MCAO) was performed according to the method described by R. Schmid-Elsaesser et al. (Stroke. 1998; 29(10): 2162-70). Test compounds were administered intravenously 20 minutes prior to reperfusion and then twice daily for six consecutive days thereafter. On Study Day 2 (one day after surgery) and Study Day 7 (7 days after surgery and before study termination), the altered Neuro...
Embodiment 2
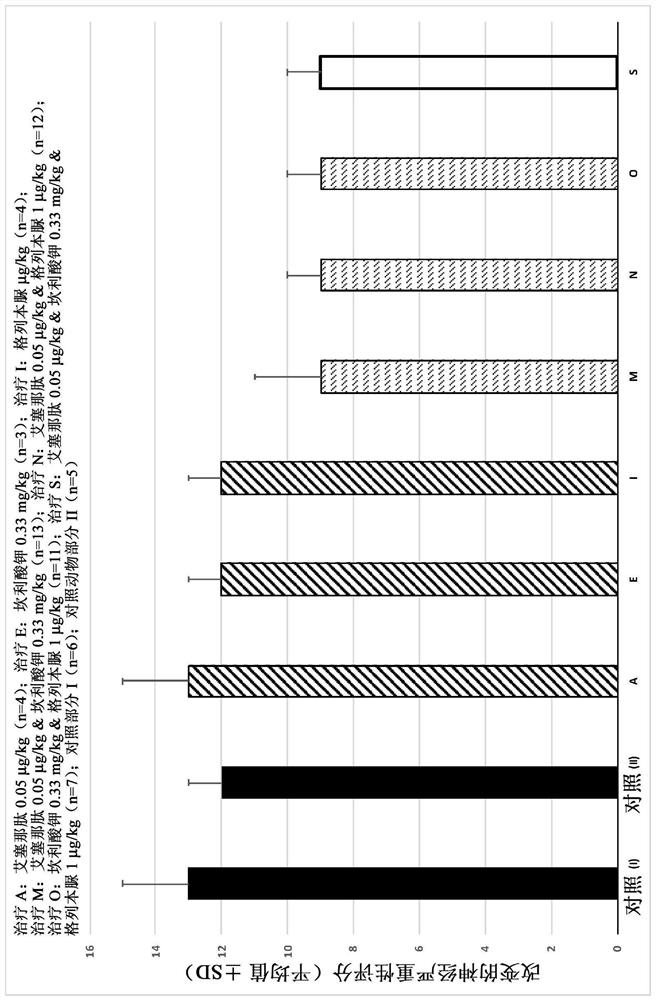
[0653] Example 2. Efficacy study of the combination of exenatide, canrenoate potassium and glibenclamide in a rat model of vascular dementia
[0654] The chronic cerebral hypoperfusion model in Wistar rats induces brain damage in the rat brain by permanently occluding both common carotid arteries, which also causes cognitive deficits. This model is similar to that of vascular dementia, and the technique can reduce blood flow by as much as 40% to 80% for several months in the cerebral cortex and hippocampus, which induces certain learning disabilities.
[0655] Research purposes
[0656] The aim of this study was to evaluate the neuroprotective efficacy of the combination of exenatide, canrenoate potassium, and glibenclamide, 24 hours after permanent ligation of both common carotid arteries, using the Vestal rat model of vascular dementia The combination was administered intravenously and then twice daily for three weeks.
[0657] therapy group
[0658] The treatment groups ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com