Drug for treating or preventing duplication of hypertension with serum hyperuricemia and/or hypercholesterolemia
A technology for hypercholesterolemia and hyperuricemia, applied in the field of drugs used to treat hypertension combined with serum hyperuricemia and/or hypercholesterolemia, and can solve problems such as lowering cholesterol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
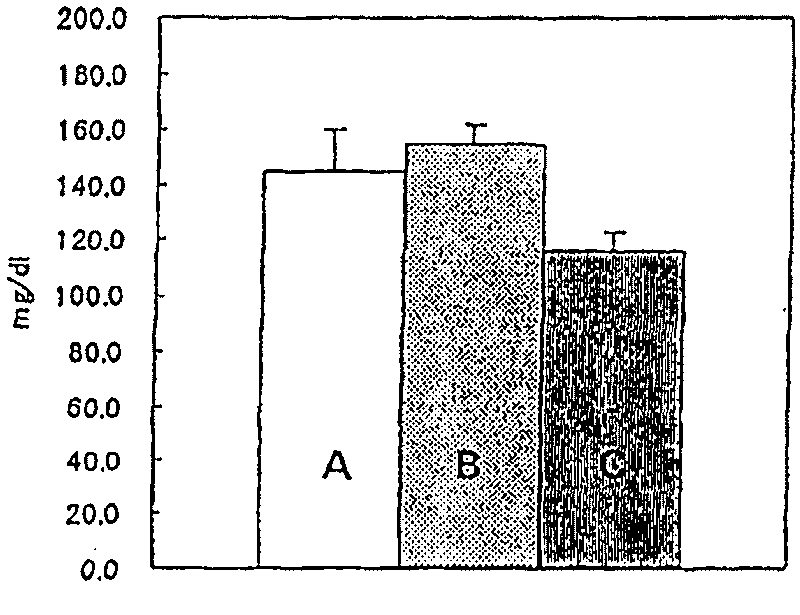
[0023] Regarding the uric acid excretion-promoting action, the delay in disappearance of phenol red in blood was used as an index to evaluate. Specifically, for 6-week-old SD male rats, 0.5% methylcellulose (MC), 30 mg / kg or 100 mg / kg of prasartan was orally administered, and 75 mg of prasartan was administered intravenously 3 hours after administration. / kg of phenol red. One hour after the administration of phenol red, blood was collected from the abdominal aorta under ether anesthesia, and the amount of phenol red in the serum was measured. The amount of phenol red in the blood serum of the control group to which prasartan was not administered was regarded as 100%, and the delayed rate of disappearance of phenol red in the blood by administration of prasartan was evaluated as the uric acid excretion promotion rate (%) . Table 1 shows the composition of the groups, the test substances and their doses, and the uric acid excretion promotion rate (mean ± standard deviation). ...
Embodiment 2
[0027] The test was carried out on patients with mild and moderate essential hypertension. Prasartan was administered to 17 patients who also suffered from hyperuricemia, and the serum uric acid values (mg / dL) (mean ± standard deviation) before administration and on the last day of administration were studied. The results are shown in Table 2. It should be noted that 40 mg / day to 160 mg / day of prasartan is administered orally at intervals of 4 weeks, and the dose is increased by increasing the dose if the antihypertensive effect is not obvious.
[0028] Table 2
[0029]
Embodiment 3
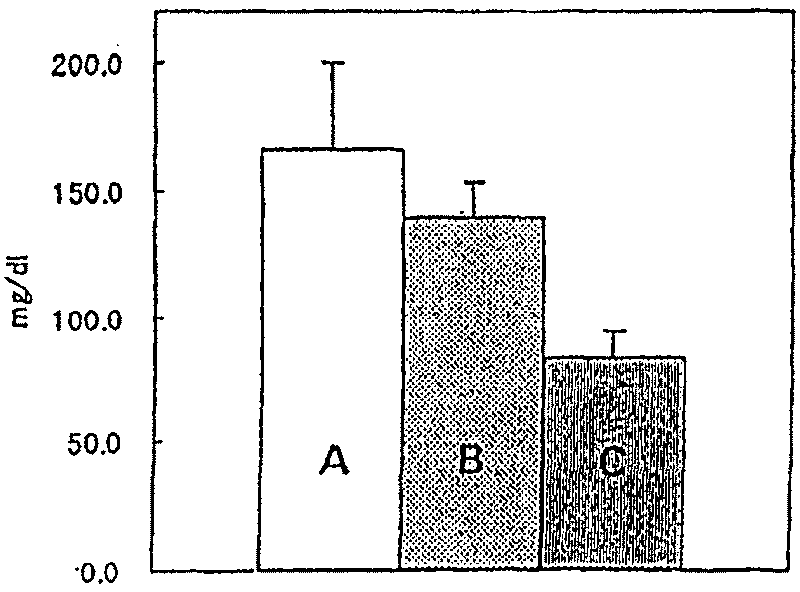
[0031] Using the Tail cuff method, the systolic blood pressure of 20-week-old male SHR (Spontaneously hypertensiverat, SPF grade, production unit; Hoshino Experimental Animal Breeding Institute: spontaneously hypertensive rats) was measured, and the animals were divided into 4 groups. The average value of the systolic blood pressure of animals in each group was uniform. Oral administration of 0.5% methyl cellulose (MC) or a solution formed by suspending the test substance in 0.5% methyl cellulose, once a day, for 28 days, before the start of administration and at the 1st, 7th, and 1st days of administration. Systolic blood pressure 5 hours after administration on the 14th and 28th. Table 3 shows the composition of the group, the test substance and its dosage. In addition, see Table 4 for the measured blood pressure (mmHg) (mean ± standard deviation) of the measurement results. It should be noted that the test substances are divided into hydrochlorothiazide (HCTZ), prasartan, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com