Method for removing residual host DNA and host protein in encephalitis B vaccine product
A technology for host proteins and vaccines, applied in the field of virus vaccine product purification, can solve problems such as adverse reactions, causing cancer, side effects, etc., and achieve the effect of improving product quality standards and breaking through quality bottlenecks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
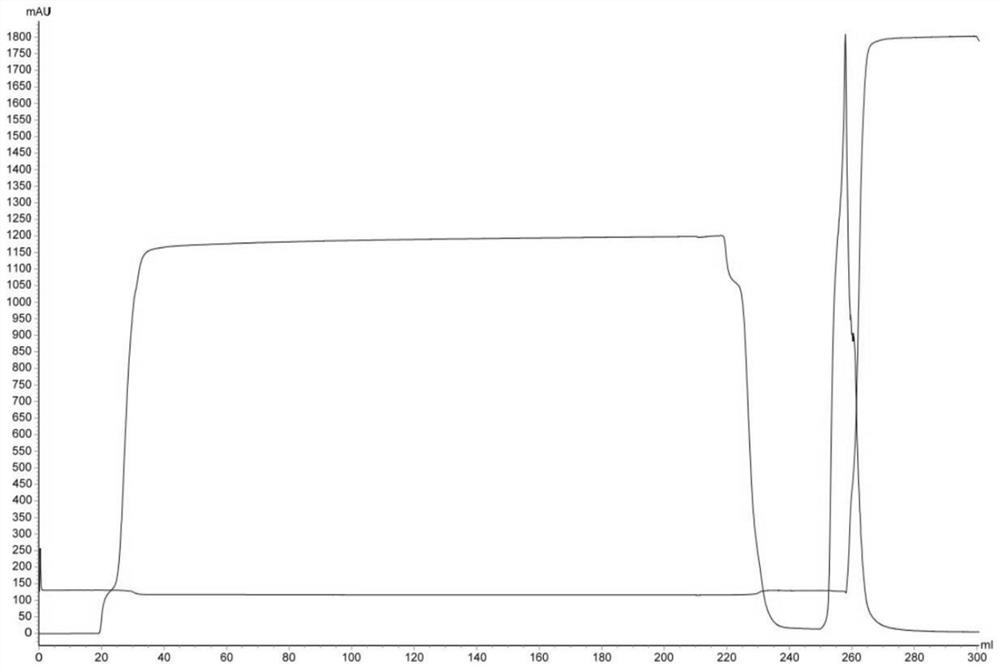
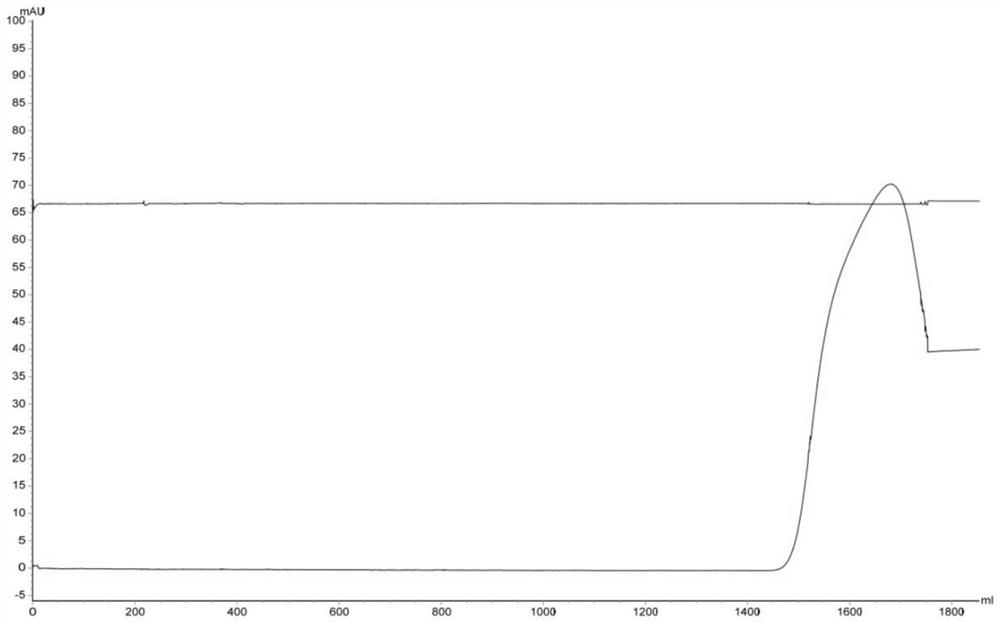
[0031] Use AKTA avant 150 automatic chromatography equipment to pass 200mL JE vaccine concentrated inactivation solution (code A1) through the XK16 chromatography column for multi-mode Capto MMC chromatography, the ultraviolet detection wavelength is 280nm, the flow rate is 1.49cm / min, and the mobile phase 0.01mol / L PBS buffer solution (pH7.5, NaCl 0.15mol / L), collect the first absorption peak, that is, the one-step purification solution of Japanese encephalitis virus protein (number is A2, see figure 1). Then one-step purified solution was subjected to Sepharose 6 Fast FLow gel filtration chromatography, the ultraviolet detection wavelength was 280nm, the flow velocity was 25cm / h, and the mobile phase was 0.01mol / L PBS buffer solution (pH7.5, NaCl0.15mol / L), and collected The first absorption peak is the final purified solution of JEV protein (numbered A3, see figure 2 ).
[0032] A1 and A3 were tested for antigen content, DNA residue, and host protein residue, respectivel...
Embodiment 2
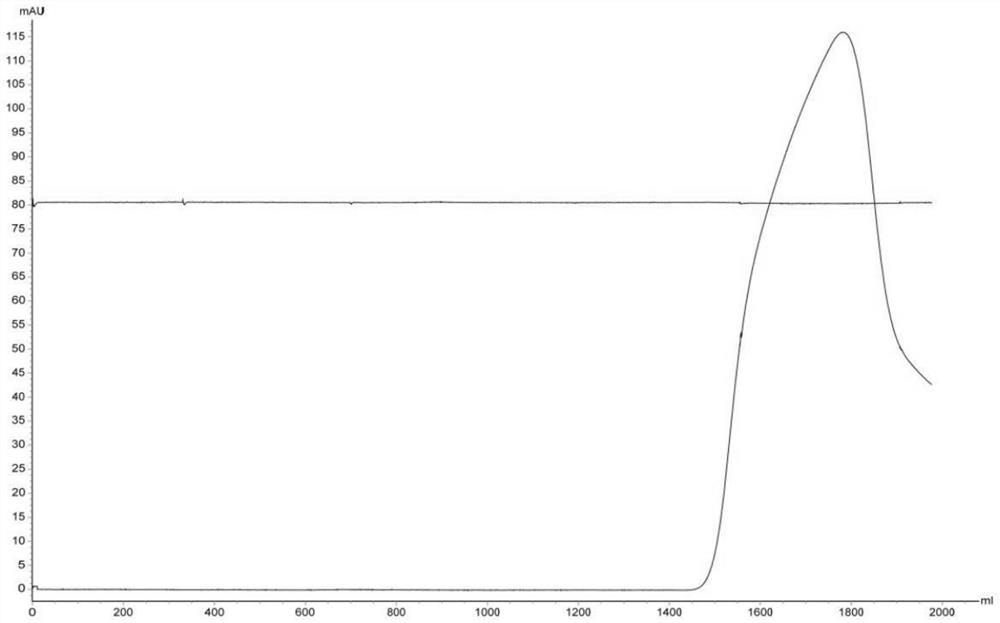
[0038] Use AKTA avant 150 automatic chromatography equipment to pass 320mL JE vaccine concentrated inactivation solution (code B1) through the XK50 chromatography column for Sepharose 6 Fast FLow gel filtration chromatography, the ultraviolet detection wavelength is 280nm, and the flow rate is 25cm / h. The mobile phase is 0.01mol / L PBS buffer (pH7.5, NaCl 0.15mol / L), and the first absorption peak is collected, which is the one-step purification solution of Japanese encephalitis virus protein (numbering is B2, see image 3 ). Then the one-step purified solution (100mL) was subjected to multi-mode CaptoMMC chromatography, the ultraviolet detection wavelength was 280nm, the flow rate was 1.49cm / min, and the mobile phase was 0.01mol / L PBS buffer solution (pH7.5, NaCl 0.15mol / L), and collected The first absorption peak is the final purified solution of JEV protein (numbered as B3, see Figure 4 ).
[0039] B1 and B3 were tested for antigen content, DNA residue, and host protein re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com