Method for inducing direct reprogramming of urine cells into renal progenitor cells and pharmaceutical composition for preventing or treating renal cell injury disease comprising renal progenitor cells reprogrammed by same method
A urine cell, reprogramming technology, applied in drug combinations, urinary system diseases, urinary tract/kidney cells, etc., can solve problems such as expanding kidney damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1: Isolation of urine cells from urine
[0097] The method for isolating urine cells from urine is based on the technique developed by Sutherland and Bain in England in 1972, as follows.
[0098] First, 1000 g of urine provided by the donor is centrifuged for 10 minutes. After removing the supernatant, the pellet remaining in the lower layer was diluted in 20 mL of PBS solution containing antibiotics such as 1% penicillin / streptomycin / amphotericin B. Then, the diluted PBS + pellet solution was centrifuged at a total of 1000g for 10 minutes. After removing the supernatant, dilute the remaining pellet in the lower layer with 1 mL of DMEM / F12-based basal medium containing 1% penicillin / streptomycin / amphotericin B, 1% L-glutamine, and 10% FBS, and seeded in gelatin-coated 12-well cell culture plates. After that, after adding 1 mL of basal medium and culturing for 3 days, the resulting cells were cultured by changing the basal medium to a growth medium prepared by...
Embodiment 2
[0099] Example 2: Introduction of reprogramming factors into urine cells
[0100] Using the retroviral packaging cell line 293GPG derived from human 293, by introducing a combination of reprogramming factors into the vectors pMXs-Oct4, pMXs-Klf4, pMXs-Sox2, pMXs-cMyc, pMXs-Slug, pMXs-Six1, pMXs-Six2 , pMXs-Osr1, pMXs-Pax2 and pMXs-Eya1 to prepare the vector, the vector pMXs-Oct4, pMXs-Klf4, pMXs-Sox2, pMXs-cMyc, pMXs-Slug, pMXs-Six1, pMXs-Six2, pMXs-Osr1 , pMXs-Pax2 and pMXs-Eya1 were prepared by inserting the nucleotide sequences encoding Oct4, Klf4, Sox2, c-Myc, Slug, Six1, Six2, Osr1, Pax2 and Eya1 proteins into pMXs vector respectively. Using Lipofectamine 2000 (Life Technologies), the reprogramming factor-introduced combination vector was injected into the urine-derived cells isolated and cultured in Example 1 to prepare reprogramming factor-introduced urine cells.
[0101] Urine cells that introduce reprogramming factors are based on a 1:1 mixed medium of DMEM and REGM ...
Embodiment 3
[0102] Example 3: Induction of urine-derived reprogrammed renal progenitor cells
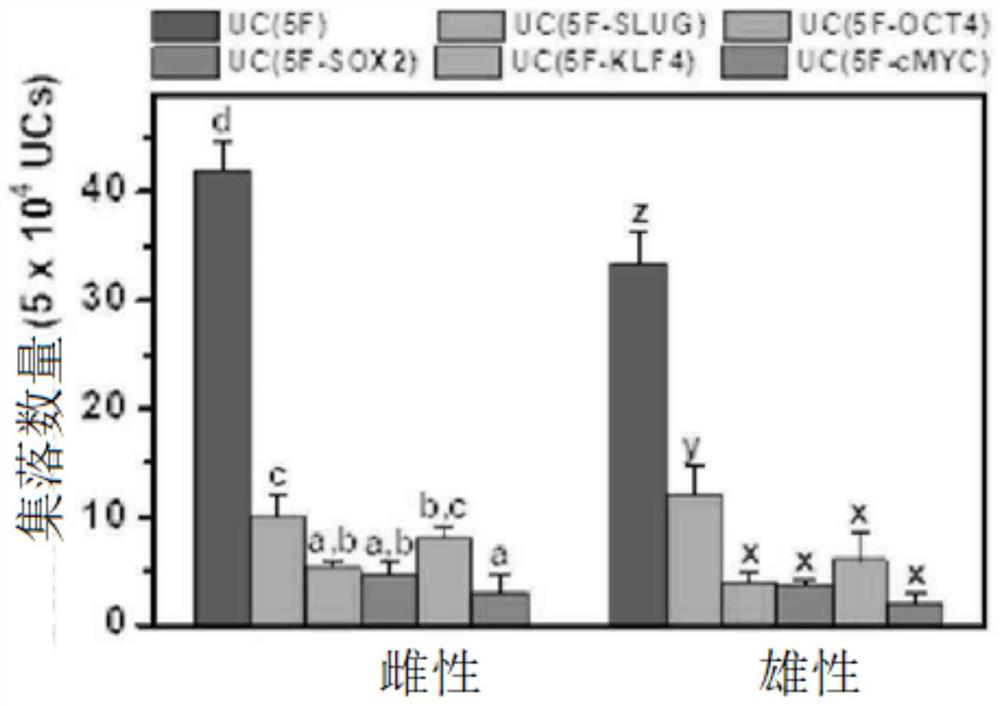
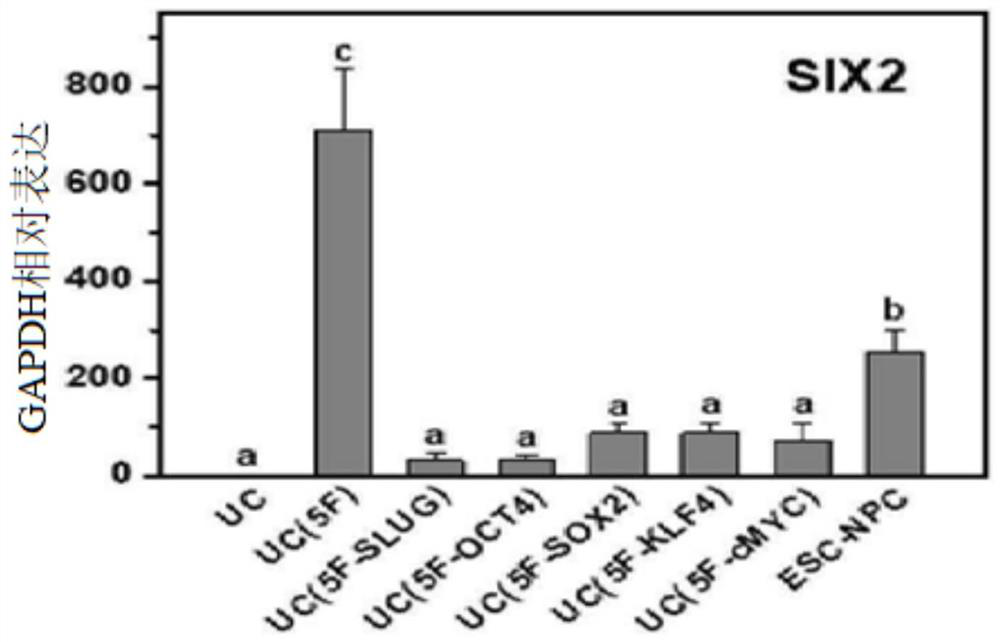
[0103] The urine-derived cells introduced with reprogramming factors were seeded on Matrigel-coated cell culture plates, and then added to Advanced RPMI 1640 medium (Gibco) containing 1 μg / ml heparin, 125 nM LDN-193189 and 0.5% L-glutamine. In the renal progenitor cell induction and expansion medium supplemented with 100ng / ml FGF9, 30ng / ml BMP7, 1.25μM CHIR99021 and 10μM Y-27632, cultured for 10 to 13 days. Upon completion of induction, the generation of colonies of renal progenitor cells could be confirmed (Figure 1A and figure 2 ).
[0104] In particular, it was confirmed that the combination of Yamanaka factor (Oct4, Klf4, Sox2, and c-Myc) to which Slug was added (5F: Oct4, Klf4, Sox2, c-Myc, and Slug) has excellent self-renewal ability and colony-forming ability (Figure 1B). However, male- and female-derived renal progenitor cells induced by the four-factor combination in which each facto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com