Two triterpenoids in sabia parviflora and preparation method thereof
A technology for compounds and triterpenoids, which is applied to two triterpenoids in C. floridarum and the field of preparation thereof, can solve the problems of restricting the development and quality control of medicinal materials of C. floridarum and their preparations, and achieves less impurities and harm to human body. The effect of small sex and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of 2 kinds of triterpenoids in the little flower Qingfengteng
[0036] 1. Experimental method
[0037] 1) Extraction:
[0038] Take the dried stems and leaves of Teng japonica, crush them, add 5 times the weight of 95% ethanol, ultrasonically extract 3 times at a temperature of 60°C, each time for 4 hours, filter, combine the extracts, and concentrate the extracts to the total Extract one-tenth of the volume to obtain concentrate I;
[0039] Add an equal volume of purified water to the concentrated solution, and stir evenly to obtain a prepared solution, and place it overnight at room temperature, filter the prepared solution overnight with a Buchner funnel, collect the filtrate, and concentrate the filtrate to alcohol-free Taste, to obtain concentrated solution II;
[0040] 2) Extraction:
[0041] Dilute the concentrated solution II in step 1) by adding purified water twice its volume to obtain a diluted solution;
[0042] Extracting ...
Embodiment 2
[0051] Embodiment 2: screening experiment
[0052] 1. Experimental method
[0053] 1.1 Thin-layer chromatography was performed on S1 and S2 respectively, developed under the condition of dichloromethane: cyclohexane = 3:1, and the color was developed by spraying vanillin concentrated sulfuric acid.
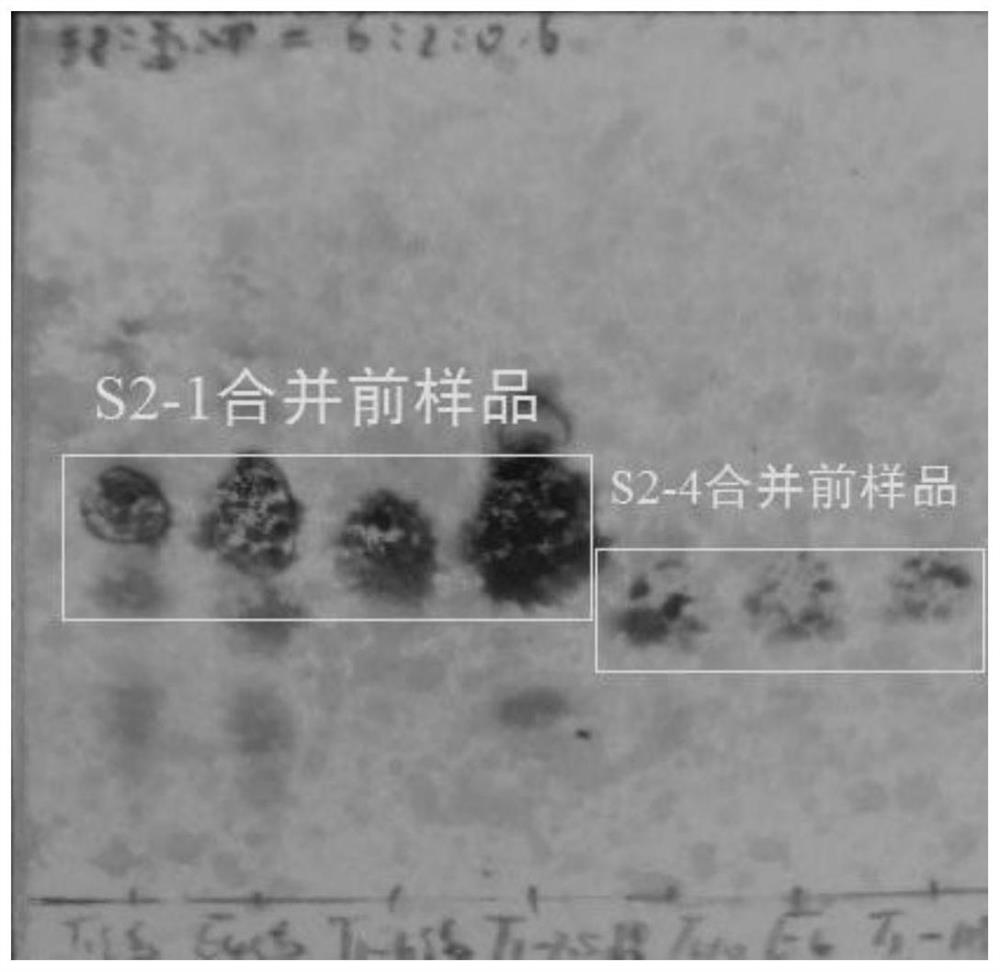
[0054] 1.2 S2-1, S2-2, S2-3, and S2-4 were combined before thin-layer chromatography, and developed under the condition of cyclohexane: dichloromethane: methanol = 6:2:0.6, using vanillin Concentrated sulfuric acid spray color.
[0055] 2. Experimental results
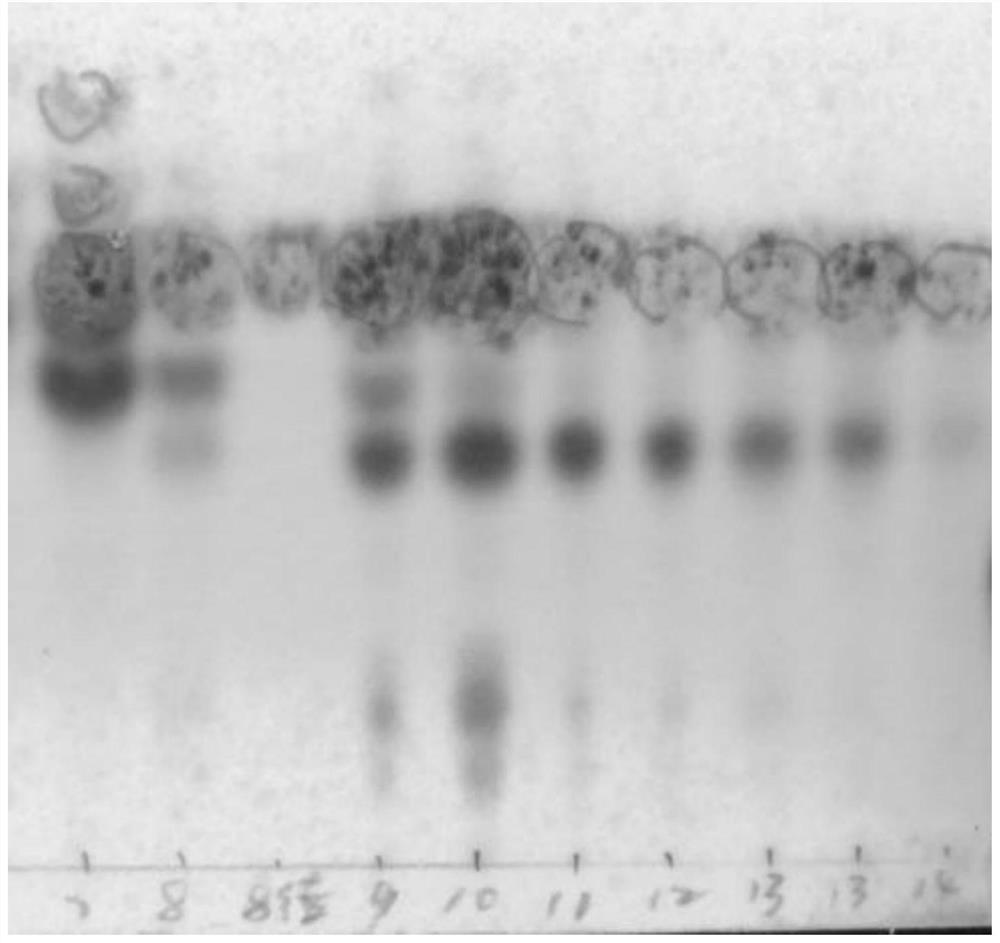
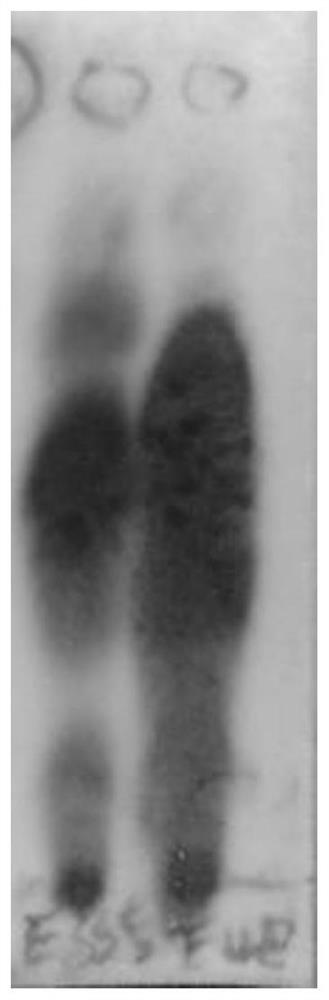
[0056] 2.1 as figure 1 and figure 2 As shown, S1 has more mixed impurities and less purple-red spots, while S2 has purple-red spots in the range of Rf value 0.2-0.4, and this part of the sample has strong crystallinity, but the purity is low, and further silica gel column chromatography is required. Separation, so S2 was selected for the next step of purification.
[0057] 2.2 as image 3 As shown, a series of purp...
Embodiment 3
[0058] Example 3: Structural Identification
[0059] 1. Experimental method
[0060] The structures of compound 1 and compound 2 were identified by carbon NMR spectroscopy.
[0061] 2. Experimental results
[0062] like Figure 4 Shown, compound 1: white crystals (chloroform). 13 C-NMR (150MHz, CDCl 3 )δ: 38.03(C-1), 27.23(C-2), 79.06(C-3), 40.25(C-4), 54.24(C-5), 18.45(C-6), 32.48(C-7 ), 38.9(C-8), 54.88(C-9), 36.71(C-10), 125.29(C-11), 125.77(C-12), 138.1(C-13), 42.36(C-14) ,24.45(C-15),35.31(C-16),34.68(C-17),133.38(C-18),36.71(C-19),33.09(C-20),36.17(C-21), 38.03(C-22), 27.9(C-23), 15.09(C-24), 16.6(C-25), 17.96(C-26), 20.25(C-27), 25.42(C-28), 32.48 (C-29), 24.14 (C-30). The above data are consistent with the literature reports, so compound 1 was identified as 3β-hydroxyl-11,13(18)-oleanadiene;
[0063] like Figure 5 Shown, compound 2: white crystals (chloroform). 13 C NMR (150MHz, CDCl 3 )δ: 37.15(C-1), 28.27(C-2), 78.73(C-3), 38.94(C-4), 51.16(C-5), 18.37(C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com