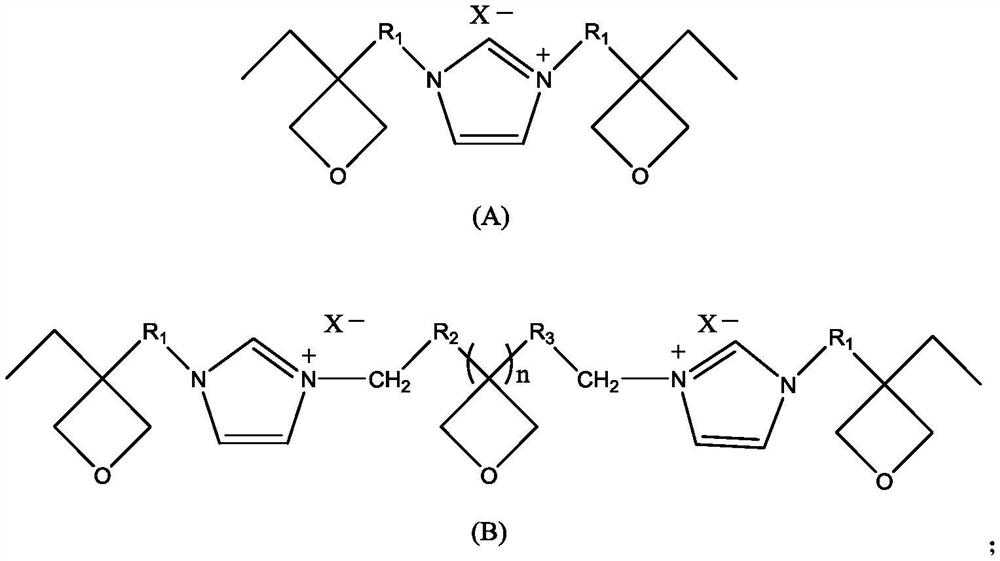
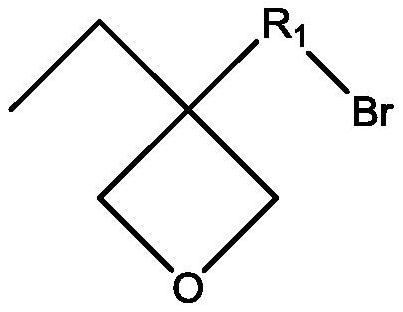
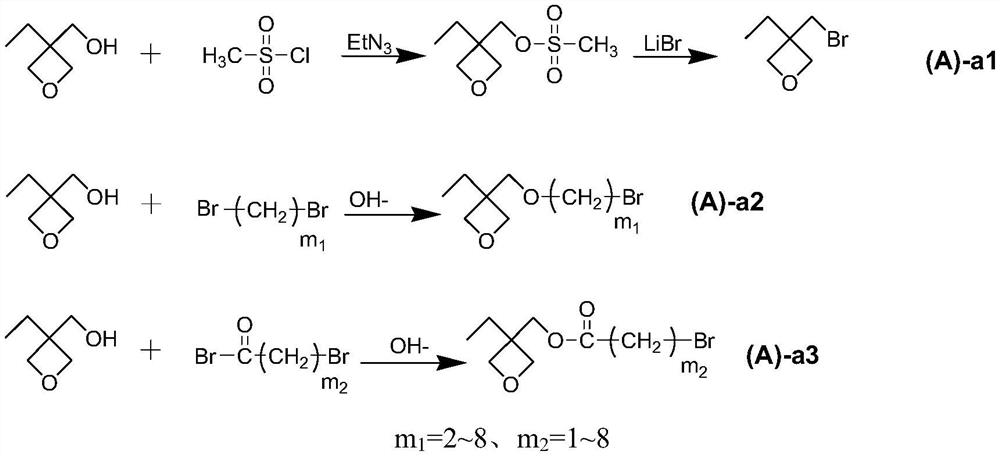
Ionic liquid containing cationic polymerizable group as well as preparation method and application of ionic liquid
A technology of ionic liquids and cations, applied in the field of light curing, can solve the problems that ionic liquids cannot meet the needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Anion is the preparation process of the ionic liquid A-1-Br of bromide ion:
[0065]
[0066] (1) In a glass flask with a stirring device and a thermometer, add 3-hydroxymethyl-3-ethyloxetane (5.8g, 50mmol), triethylamine (6.07g, 60mmol) and 200ml of toluene , keep the temperature at 5-10°C, slowly add methanesulfonyl chloride (6.30g, 55mmol), and react at this temperature for 2 hours under stirring; then add lithium bromide (4.78g, 55mmol) to the glass bottle, and heat up to 60 After reacting at ~70°C for ten hours, a certain amount of lithium bromide (1.26 g, 10 mmol) was added, and the reaction was continued at this temperature for 8 hours. After the reaction was completed, the liquid was separated, and the obtained organic layer was distilled under reduced pressure to obtain the intermediate product (A)-a1-1;
[0067]
[0068] (2) In an anhydrous and oxygen-free system, dissolve imidazole (3.40g, 50mmol) in 20ml of anhydrous tetrahydrofuran, control the react...
Embodiment 2
[0070] Anionic Bis(trifluoromethane)sulfonylimide Ionic Liquid A-1-NTf 2 The preparation process:
[0071] The ionic liquid product (1.73g, 5mmol) of the bromide ion obtained in Example 1 was dissolved in 100mlH at 80°C 2 O, add 10ml of LiNTf 2 (17.21g, 60mmol) aqueous solution, react overnight at room temperature. After the reaction, extract with 50ml dichloromethane and wash with water, add anhydrous MgSO 4 Drying, evaporating the volatile part, and finally obtaining the anion as the product A-1-NTf of bis(trifluoromethane)sulfonylimide 2 .
Embodiment 3
[0073] Anion is the preparation process of the ionic liquid A-3-Br of bromide ion:
[0074]
[0075] (1) Add 3-hydroxymethyl-3-ethyloxetane (5.8g, 50mmol) to a three-necked round-bottomed flask containing 100ml of dichloromethane, cool the mixture to 0°C, and add triethylamine (6.07g, 60mmol) and 4-dimethylaminopyridine (1.22g, 10mmol), stirred for 10-60 minutes and then added 2-bromopropionyl bromide (10.01g, 60mmol). After the reaction, the mixture was washed with 100ml saturated NaHCO 3 solution washing, adding anhydrous MgSO 4 After drying, the organic solvent was evaporated and the residue was purified by column chromatography (ethyl acetate:hexane=2:1) to obtain intermediate product A-a3-1.
[0076]
[0077](2) In an anhydrous and oxygen-free system, dissolve imidazole (3.40g, 50mmol) in 20ml of anhydrous tetrahydrofuran, control the reaction temperature to be 0°C, and add the imidazole solution in anhydrous tetrahydrofuran dropwise to NaH( After 2.4g (60% min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com