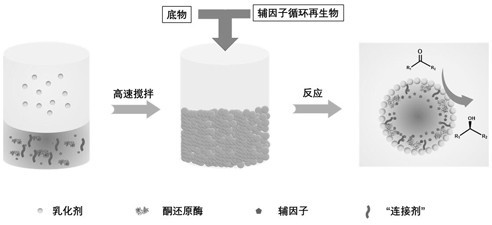
Pickering emulsion and method for preparing chiral alcohol compound based on enzyme catalysis of Pickering emulsion
A compound and emulsion technology, applied in the field of enzyme catalysis, can solve the problems of cofactor loss, loss, and inability to efficiently recycle and regenerate cofactors, and achieve the effects of improved reaction conversion, efficient synergistic catalysis, and efficient preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
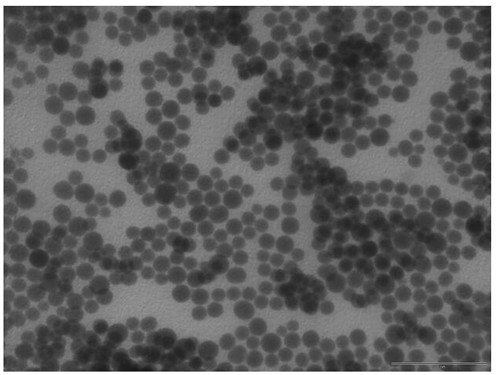
[0032] 1) After drying 1.0 g SiO 2 Nanoparticles (about 60nm in size) were dispersed into 12 mL of toluene, 0.6 g of octyltrimethoxysilane and 0.12 g of triethylamine were added, and the o C and reflux for 4 hours under stirring under nitrogen protection conditions. After centrifugation, washing and drying, the interface active nano-SiO 2 (shape like figure 2 As shown, the diameter of the Pickering emulsion microdroplets is 5-300 μm).
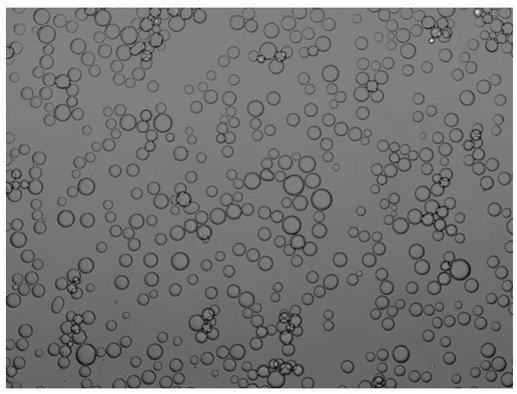
[0033] 2) Mix 0.36 mL of ketoreductase solution (ketoreductase selected from one of ES-KRED-101~ES-KRED-287 and its mutants), 0.056mg NADP + , 0.1mmol polyethyleneimine was added to 3.2mL100mM PBS buffer, and mixed evenly by magnetic stirring; then 0.158 g of interface-active SiO 2 Ultrasonic dispersion in 8 mL of n-heptane was added to the mixture of the above buffer and enzyme; finally, a Pickering emulsion with immobilized enzyme catalyst was formed by high-speed stirring at 5000 rpm (morphology as image 3 shown), and then suck off th...
Embodiment 2
[0036] 1) After drying 1.0 g SiO 2 Nanoparticles were dispersed into 15 mL of toluene, 0.35 g of dichlorodimethylsilane and 0.65 g of n-hexylamine were added, at 60 o C and reflux for 4 hours under stirring under nitrogen protection conditions. After centrifugation, washing and drying, the interface active nano-SiO 2 .
[0037] 2) Add 0.72 mL of ketoreductase solution, 0.112 mg of NADP + , 0.1 mmol polyacrylic acid was added to 6.4 mL of 100 mM PBS buffer, and mixed evenly by magnetic stirring; then 0.316 g of interface-active SiO 2 Ultrasonic dispersion in 16 mL of n-heptane and added to the mixture of the above buffer and enzyme; finally by 5000 rpm high-speed stirring to form a Pickering emulsion of immobilized enzyme catalyst.
[0038] 3) Add 4mmol (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone and 4.8mmol isopropanol Mix it into the Pickering emulsion mentioned above, and place it at 30 o C water bath was left to react for 12h, then the oil pha...
Embodiment 3
[0040] 1) After drying 1.2 g SiO 2 Nanoparticles were dispersed into 20 mL of toluene, 0.48 g of dichlorodimethylsilane and 1.0 g of n-hexylamine were added, at 60 o C and reflux for 5 hours under stirring under nitrogen protection conditions. After centrifugation, washing and drying, the interface active nano-SiO 2 .
[0041] 2) Mix 0.18 mL of ketoreductase solution, 0.028 mg NADP + , 0.1 mmol polyacrylic acid was added to 3.2 mL of 100 mM PBS buffer, and mixed evenly by magnetic stirring; then 0.158 g of interface-active SiO 2 Ultrasonic dispersion in 8mL of n-heptane and added to the mixture of the above buffer and enzyme; finally by 5000 rpm high-speed stirring to form a Pickering emulsion of immobilized enzyme catalyst.
[0042] 3) Add 4 mmol of acetophenone and 4.8 mmol of isopropanol to the above Pickering emulsion and mix well, place at 30 o C water bath was left to react for 12 h, then the oil phase was added to extract the product, 4 mmol acetophenone and 4.8 mm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com