Therapeutic agent for polycythemia
A polycythemia, antibody technology, applied in extracellular fluid diseases, drug combinations, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve problems such as low satisfaction and lower QOL of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1: Identification of the binding site of the TfR436 antibody
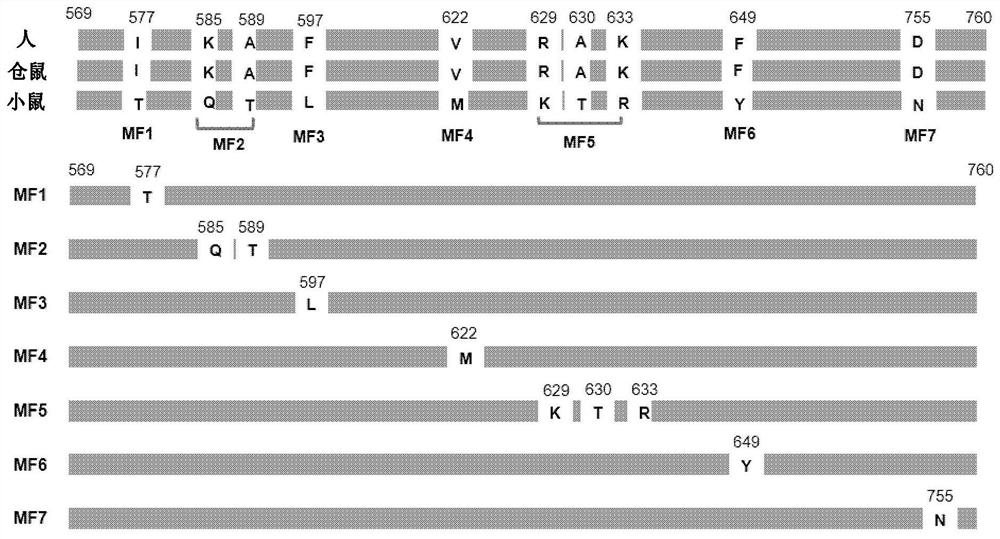
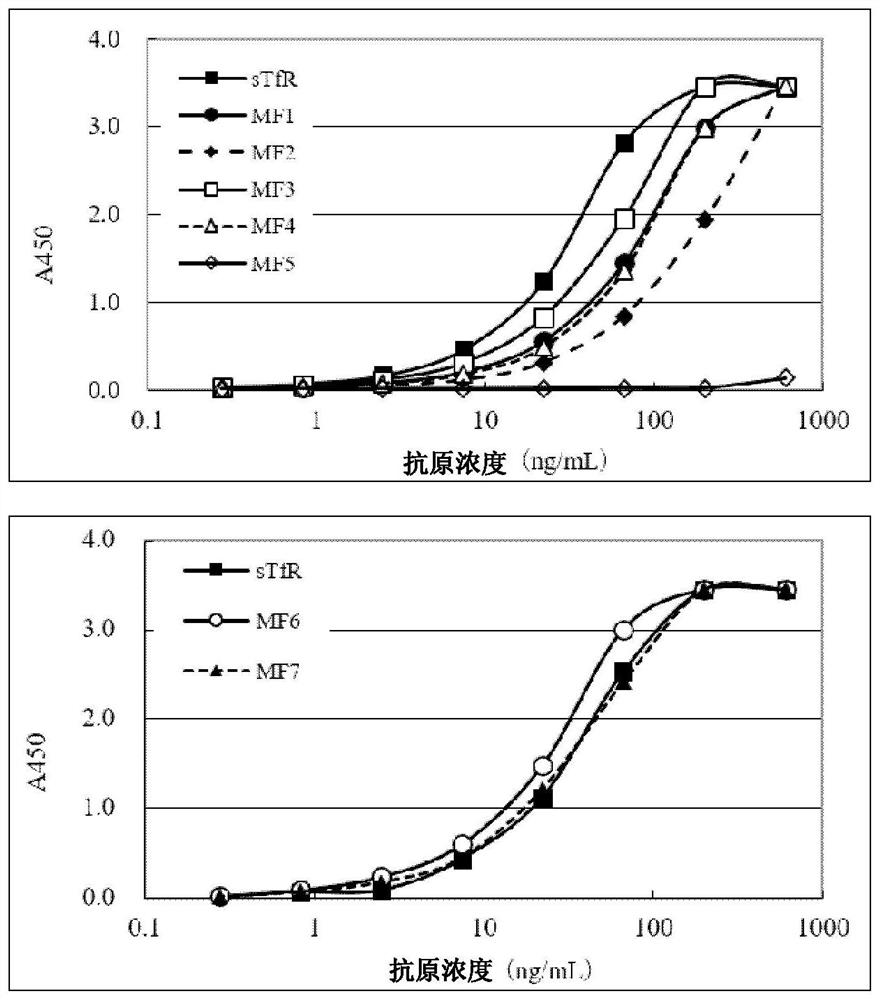
[0112] The TfR436 antibody does not cross-react with mouse TfR, but shows cross-reactivity with hamster TfR. The amino acid sequences of the transferrin (TF) binding site (amino acids 569-760) in TfR were compared. Amino acids in the human TfR sequence identical to those of hamster but different from those of mouse were selected. Such as figure 1 The selected amino acids were subjected to point mutations as shown to prepare soluble TfR mutant fragments (TfR mutant fragments). (1) Preparation of soluble wild type TfR (wild type TfR, sTfR) and TfR mutant fragment (TfR mutant fragment) (MF1-MF7)
[0113] Fully synthetically encoded human TfR extracellular region (amino acids 89-760) or figure 1 The nucleotide sequences of the respective TfR mutant fragments (MF1 to MF7) and AAARGGPEQKLISEE DLNSAVDHHHHHH (SEQ ID NO: 10) are shown. The neomycin resistance gene and the DHFR gene were introduced into t...
Embodiment 2
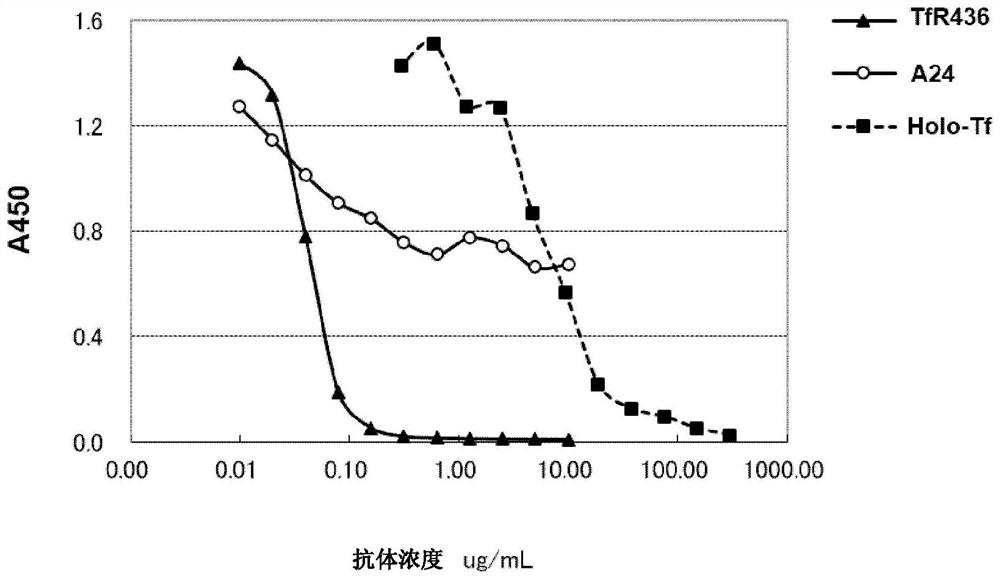
[0117] Example 2: Comparison of Tf-TfR Binding Inhibition of TfR436 Antibody and Comparative Antibody
[0118] (1) Production of comparative antibody A24
[0119] The A24 antibody against TfR is described in the patent document US2008 / 0193453. In order to compare the TfR436 antibody with this antibody, the deposited hybridoma (Hybridoma) was obtained to produce the antibody. Specifically, hybridomas were added to RPMI1640 (GIBCO), FBS 10% medium so that the cell concentration became 1-2×10 5 / mL at 37°C in 5% CO 2 Cultivate in an incubator. After the expanded culture, the cells were recovered by centrifugation, washed twice with PBS, and further expanded to 550 mL in a serum-free medium cosmedium005 (Cosmobio) and 0.5% Nutridoma-CS (Roche). Five days after the cells became confluent, the culture supernatant was recovered by centrifugation.
[0120] The recovered supernatant was loaded on the protein A carrier (Ab-Capcher ExTra: Protenova Company), and the antibody bound t...
Embodiment 3
[0124] Example 3: EPO-independent nucleated erythroid differentiation inhibitory effect of hematopoietic stem cells derived from PV patients
[0125] Hematopoietic stem cells derived from PV patients are characterized by the ability to form BFU-E and CFU-E in the absence of erythropoietin (EPO). The inhibitory activity of TfR436 on the colony formation of these cells was investigated.
[0126] (1) PBMC separation
[0127] With regard to the peripheral blood of PV patients after bloodletting, 20 mL of peripheral blood was respectively injected into three 50 mL centrifuge tubes, and an equal amount of PBS (Gibco) was added and mixed. Inject LymphoSep and lymphocyte separation medium (Lymphocyte Separation Media, MP Biomedicals TM ) 15 mL, slowly added 30 mL each of peripheral blood diluted with PBS, and centrifuged at 1,500 rpm for 30 minutes at room temperature. After centrifugation, the plasma in the supernatant was removed, and the PBMC layer was collected with a pipette a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com