Light cleavable protein mutant with high light cleavage efficiency and application thereof
A technology of protein mutants and mutants, which is applied in the field of genetic engineering, can solve the problems of low cutting efficiency and long UV light irradiation time, and achieve the effects of improved photocutting efficiency, high-throughput protein purification, and high purification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] This example is used to illustrate the obtaining of the photocleavable protein provided by the present invention.
[0063] The pBAD-phoCl plasmid used in this example is an expression system in which the pBAD plasmid is used as a carrier to insert the phoCl expression gene. The preparation method can refer to Zhang Fan, 2012, Preparation of recombinant Bifidobacterium longum engineering bacteria expressing interferon-α2b, China Journal of Pathogen Biology, 007(003):190-191,194.
[0064] In this example, primers F1 (ATGGTGATCCCTGACTACTTCAAGCAG, SEQ ID NO: 11) and R1 (TTACCGTGGGTACTTGGTGAACACG, SEQ ID NO: 12) were synthesized by Ruibo Xingke Biotechnology Co., Ltd.
[0065] (1) Construction of photocleavable protein mutant library
[0066] Using the pBAD-phoCl plasmid as a template, using primers F1 and R1 as forward and reverse primers, respectively, the directed evolution of error-prone PCR was carried out according to the reaction system in Table 1 and the error-prone P...
Embodiment 2
[0097] This example is used to illustrate the expression and purification of the fusion protein provided by the present invention.
[0098] (1) Detection of fusion protein activity
[0099] Based on molecular biology operations, the mutant proteins 1 and 2 screened in the test (5) in Example 1 were fused with the antimicrobial peptide Hisatatin 1 for expression.
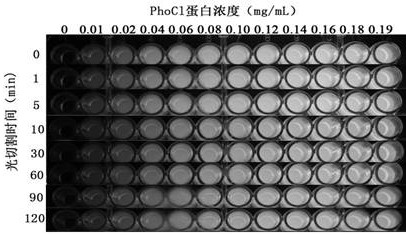
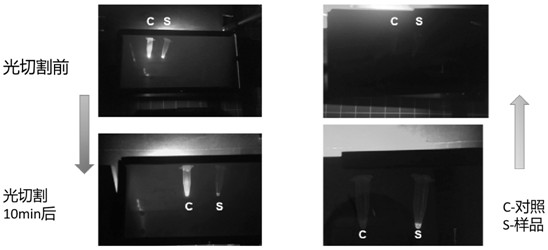
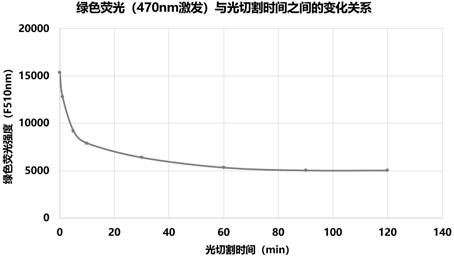
[0100] The original protein phoCl, mutant 1 and mutant 2 linked with a purification tag (polyhistidine tag, amino acid sequence shown in SEQ ID NO: 4) were fused with the antimicrobial peptide Hisatin1 (synthesized by General Biotech) , after purification using the purification tag, the purified protein was cleaved at a wavelength of 385nm for 0min, 1min, 5min, 10min, 30min, 60min, 90min and 120min, and the cleavage was detected by electrophoresis.
[0101] Figure 12-14 The antibacterial peptide fused with the original protein (phoCl-Histatin1), the antimicrobial peptide fused with mutant 1 (mutant 1-Histatin1) an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Minimum inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com