Method for catalytically synthesizing N-arylated derivative of pyrimidine-2-amine
A technology of arylation and derivatives, applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, chemical/physical processes, etc., can solve problems such as harsh reaction conditions and low catalyst conversion numbers, etc. Achieve the effects of mild reaction conditions, less by-products, and stable physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
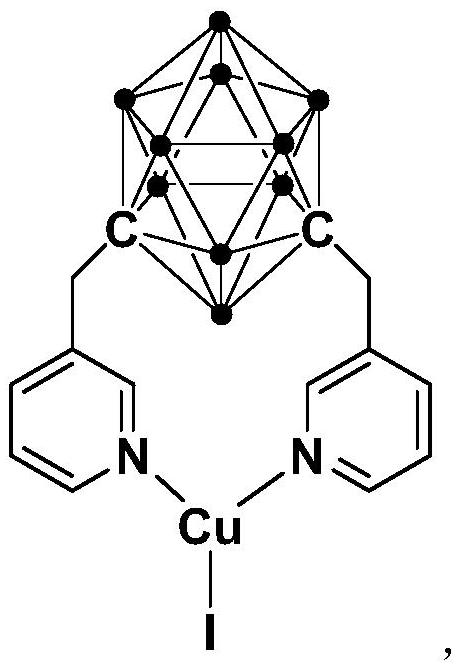
[0031] The preparation method of the cuprous complex containing the meta-carborane ligand of N-coordination comprises the following steps:
[0032] 1) Add the n-BuLi solution to the m-carborane solution at -80°C to -75°C, stir and react at room temperature for 30-60min;
[0033] 2) Add 3-chloromethylpyridine and react at room temperature for 3-5h;
[0034] 3) Add CuI, react at room temperature for 2-5h, and obtain the cuprous complex after post-treatment.
[0035] In step 1), the n-BuLi solution is n-BuLi n-hexane solution, and the m-carborane solution is m-carborane tetrahydrofuran solution.
[0036] In step 3), the post-treatment process is: standing and filtering after the reaction, decompressing to dry the solvent to obtain the crude product, and then subjecting the crude product to column chromatography separation; during the column chromatography separation process, the eluent is petroleum ether and A mixed solvent of ethyl acetate, and the volume ratio of petroleum et...
Embodiment 1
[0039] Synthesis of N,N-Coordinated Cuprous Complex Cu Containing Meta-Carborane Ligands:
[0040]
[0041] At -78°C, n-BuLi (1.6M) in n-hexane (1.00 mL, 1.6 mmol) was slowly added dropwise to m-C containing m-carborane 2 B 10 h 10 (92.0mg, 0.64mmol) in tetrahydrofuran solution, stirred at this temperature for 30 minutes, slowly rose to room temperature and continued to react for 1 hour, then added 3-chloromethylpyridine (162.3mg, 1.28mmol), and continued to react at room temperature React for 5 hours. CuI (122.9 mg, 0.64 mmol) was then added to the reaction for another 2 hours. After the reaction, stand and filter, and dry the solvent under reduced pressure, the obtained crude product is separated by column chromatography (petroleum ether / ethyl acetate=8:1), and the brown target product cuprous complex Cu (257.8mg, Yield 78%).
[0042] 1 H NMR (400MHz, CDCl 3 ,25℃):δ=7.83(d,J=7.0Hz,2H),7.55(s,2H),7.43(d,J=6.5Hz,2H),7.35(t,J=6.5Hz,2H), 2.91(s,4H). Elemental analysis...
Embodiment 2
[0044] The cuprous complex catalyzes the coupling reaction, and the specific process is as follows:
[0045]
[0046] The cuprous complex Cu (0.01mmol) and iodobenzene (1.1mmol) dissolved in N-methylpyrimidin-2-amine (1mmol), cuprous complex Cu (0.01mmol) and iodobenzene (1.1mmol) were used as a catalyst as a catalyst. In 3mL toluene, react at room temperature for 6 hours. After the end, the concentrated reaction solution is directly separated by silica gel column chromatography, dried until the mass remains unchanged, and the corresponding product C is obtained. 11 h 11 N 3 (yield 91%), 1 H NMR (400MHz, CDCl 3 ,25°C): δ=8.35(d, J=7.0Hz, 2H), 7.45(t, J=7.0Hz, 2H), 7.33(d, J=7.0Hz, 2H), 7.27(t, J=7.5 Hz, 1H), 6.56(t, J=4.0Hz, 1H), 3.52(s, 3H). Elemental analysis: C 71.33, H 5.99, N 22.69 (theoretical); C 71.36, H 5.98, N 22.75 (actual).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com