Method for preparing iron phosphate from ferro-nickel alloy
A technology of nickel-iron alloy and iron phosphate, which is applied in the field of lithium-ion battery precursors, can solve the problems that iron phosphate products do not meet industry standards, and achieve the effects of reducing S and Ni content, reducing entrainment, and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
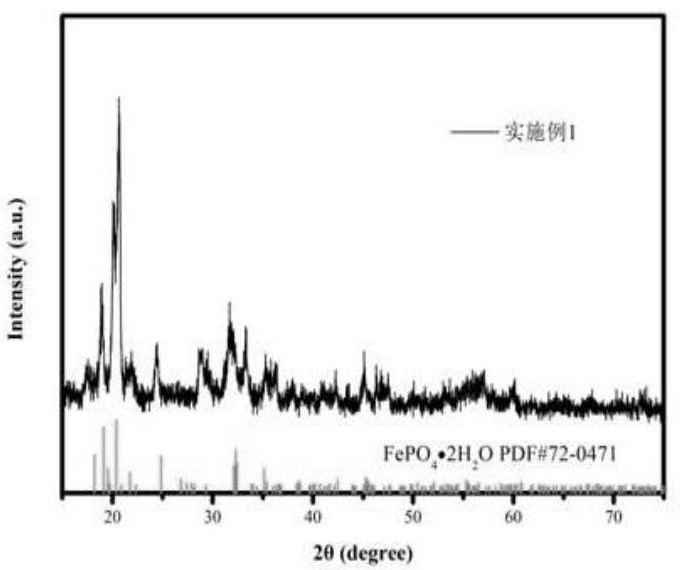
Embodiment 1
[0023] Step 1, take by weighing 80g nickel-iron alloy (Fe: 74.6%, Ni: 25.4%); Preparation concentration is 90mL of the phosphoric acid solution of 14mol / L, concentration is the sulfuric acid solution 440mL of 2mol / L; Prepared phosphoric acid solution and sulfuric acid solution Add it into the nickel-iron alloy, heat up and stir for a period of time to obtain the nickel-iron leaching solution;
[0024] Step 2, the above-mentioned ferronickel leaching solution, the concentration of 1mol / L hydrogen peroxide solution and ammonia solution are added to the reactor in parallel, the feeding rate of the control nickel-iron leaching solution is 8mL / min, and the feeding rate of hydrogen peroxide is 11mL / min. The feeding rate of ammonia water keeps the pH value of the reaction system at about 1.5, and the reaction temperature is 60°C;
[0025] Step 3. After adding the materials in step 2, the temperature of the reaction system is raised to 90° C., and kept for 2 hours;
[0026] Step 4 an...
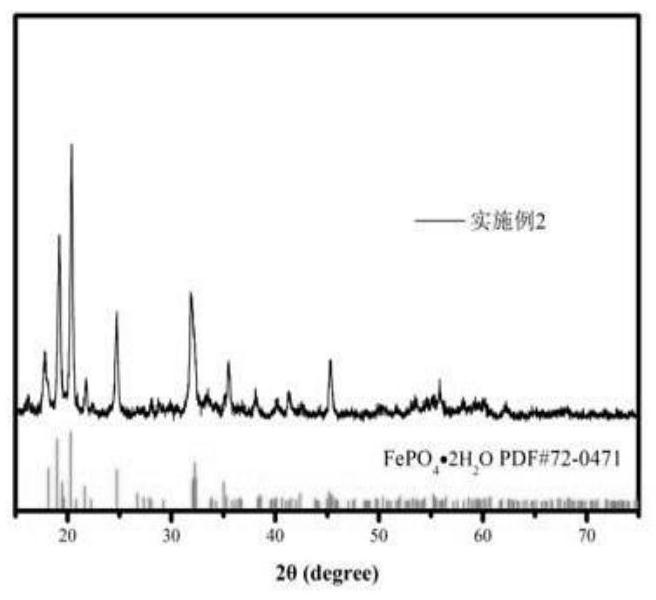
Embodiment 2
[0028] Step 1, take by weighing 80g nickel-iron alloy (Fe: 60%, Ni: 40%); Preparation concentration is 90mL of the phosphoric acid solution of 9mol / L, concentration is the sulfuric acid solution 440mL of 7mol / L; Prepared phosphoric acid solution and sulfuric acid solution Add it into the nickel-iron alloy, heat up and stir for a period of time to obtain the nickel-iron leaching solution;
[0029] Step 2, the above-mentioned ferronickel leaching solution, the concentration of 0.5mol / L hydrogen peroxide solution and ammonia solution are added to the reaction kettle in parallel, the feeding rate of the ferronickel leaching solution is controlled to be 6mL / min, and the feeding rate of hydrogen peroxide is 6mL / min, Control the feeding rate of ammonia water, keep the pH value of the reaction system at about 1.4, and the reaction temperature at 50°C;
[0030] Step 3. After adding the materials in step 2, the temperature of the reaction system is raised to 50° C. and kept for 60 minut...
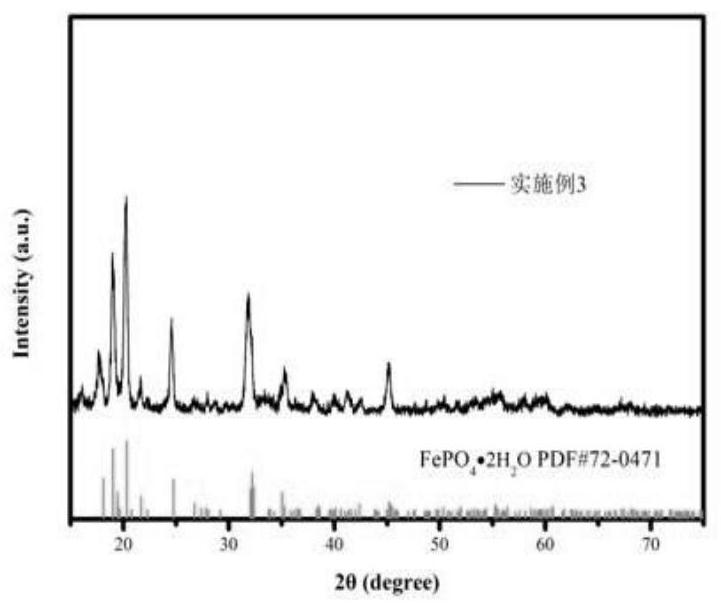
Embodiment 3
[0033] Step 1, take by weighing 80g nickel-iron alloy (Fe: 80%, Ni: 20%); Preparation concentration is 90mL of the phosphoric acid solution of 16mol / L, concentration is the sulfuric acid solution 440mL of 7mol / L; Prepared phosphoric acid solution and sulfuric acid solution Add it into the nickel-iron alloy, heat up and stir for a period of time to obtain the nickel-iron leaching solution;
[0034] Step 2, the above-mentioned ferronickel leaching solution, the concentration of 2mol / L hydrogen peroxide solution and ammonia solution are added to the reaction kettle in parallel, the feeding rate of the control nickel-iron leaching solution is 10mL / min, and the feeding rate of hydrogen peroxide is 15mL / min. The feeding rate of ammonia water keeps the pH value of the reaction system at about 2, and the reaction temperature is 70°C;
[0035] Step 3. After adding the materials in step 2, the temperature of the reaction system is raised to 120°C and kept for 180 minutes;
[0036] Step...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com