Fat component used in medical formula food for treating sarcopenia syndrome as well as preparation method and application of fat component
A technology for muscle attenuation and formula food, applied in the direction of edible oil/fat, application, food science, etc., can solve the problems of fat fatty acid ratio and function understanding, and achieve the goal of inhibiting relative expression, inhibiting inflammatory response, and improving fluidity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051]Fat components used in sarcopenia medical formula foods, including the following raw materials in weight percentages: 45% medium chain triglycerides, 12.5% sesame oil, 12.5% flaxseed oil, 12.5% krill oil, 10% Fish Oil, 7.5% Peanut Oil.
[0052] At room temperature, various oils (except medium-chain triglycerides) are added into the reactor according to the mass ratio at room temperature, mixed and stirred, the stirring time is 15min, the stirring speed is 100rpm, and the mixed oil is obtained after stirring; the heating rate is 4°C / min, heat up to 35min, stirring speed is 100rpm, add medium-chain triglyceride after stirring, continue stirring for 20min, stop stirring, then cool down to room temperature at 4°C / min, and end stirring.
[0053] The fatty acid composition and lipid accompanying substances (sesaxanol, astaxanthin, tocopherol, etc.) content of the fat components were detected, and the results are shown in Table 1 and Table 2, respectively.
[0054] Muscl...
Embodiment 2
[0057] Fat components used in sarcopenia medical formula food, including the following raw materials in weight percentage: 45% medium chain triglycerides, 15% sesame oil, 10% flaxseed oil, 10% krill oil, 15% Fish oil, 5% peanut oil, each component is mixed and stirred with various oils and fats at room temperature to obtain a finished product; the specific preparation method is the same as in Example 1.
[0058] The fatty acid composition and lipid accompanying substances (sesaxanol, astaxanthin, tocopherol, etc.) content of the fat components were detected, and the results are shown in Table 1 and Table 2, respectively.
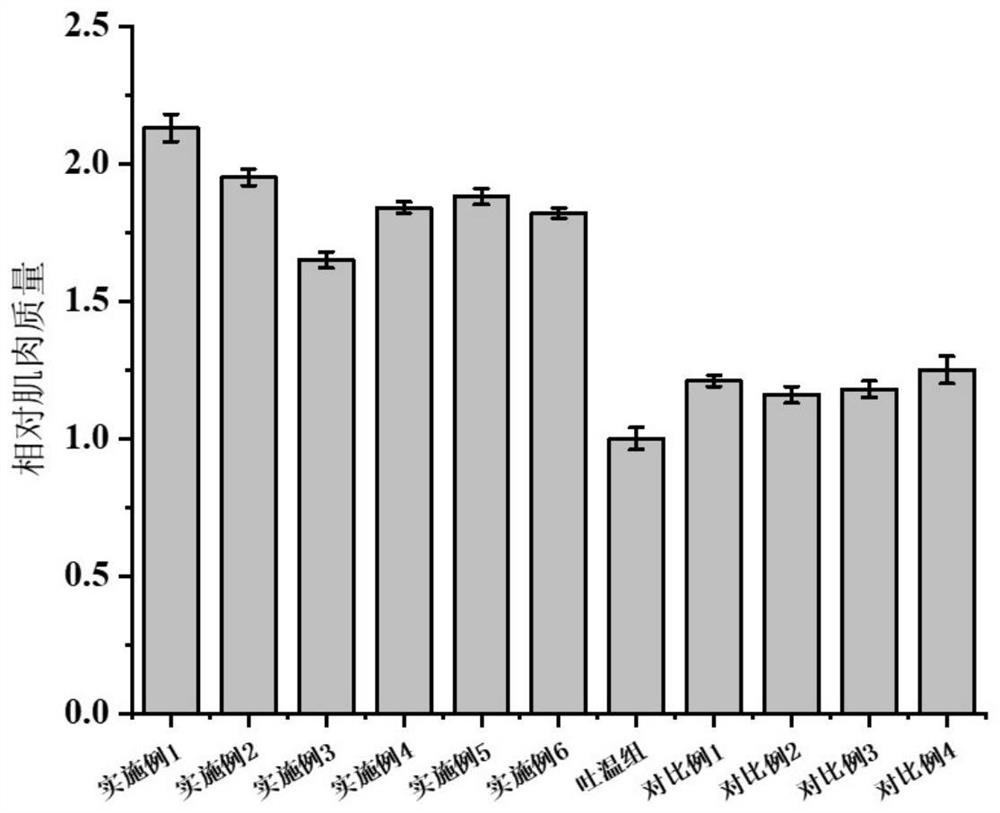
[0059] Muscle mass was assessed by the juvenile zebrafish model, as shown in figure 1 shown.
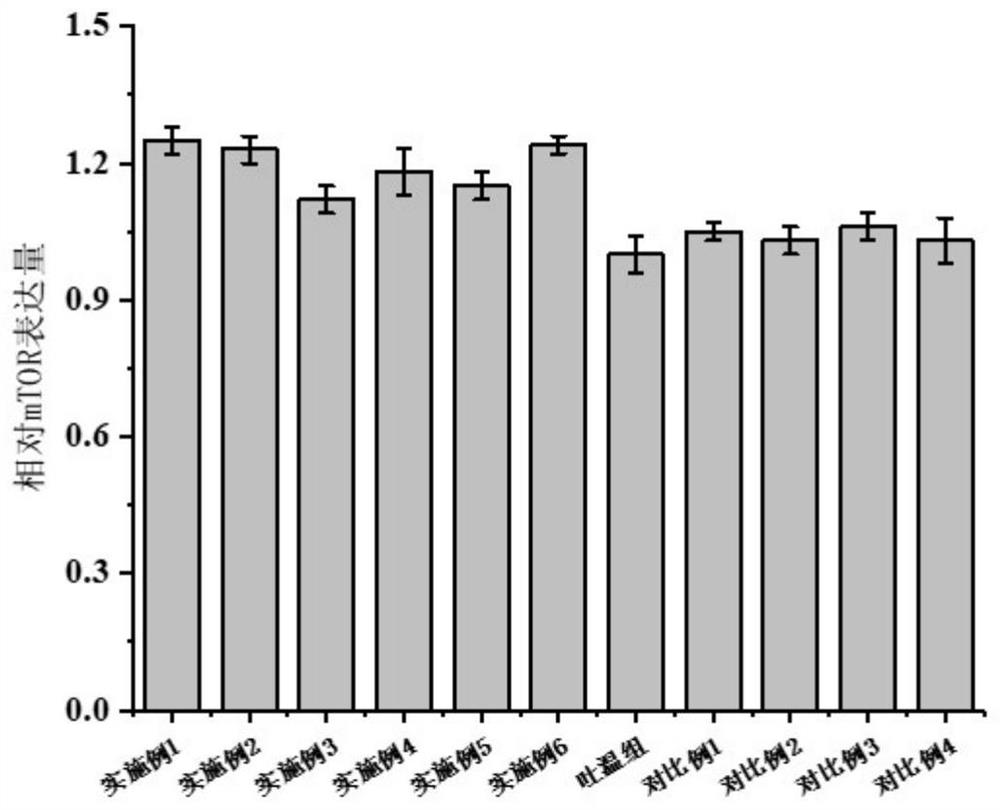
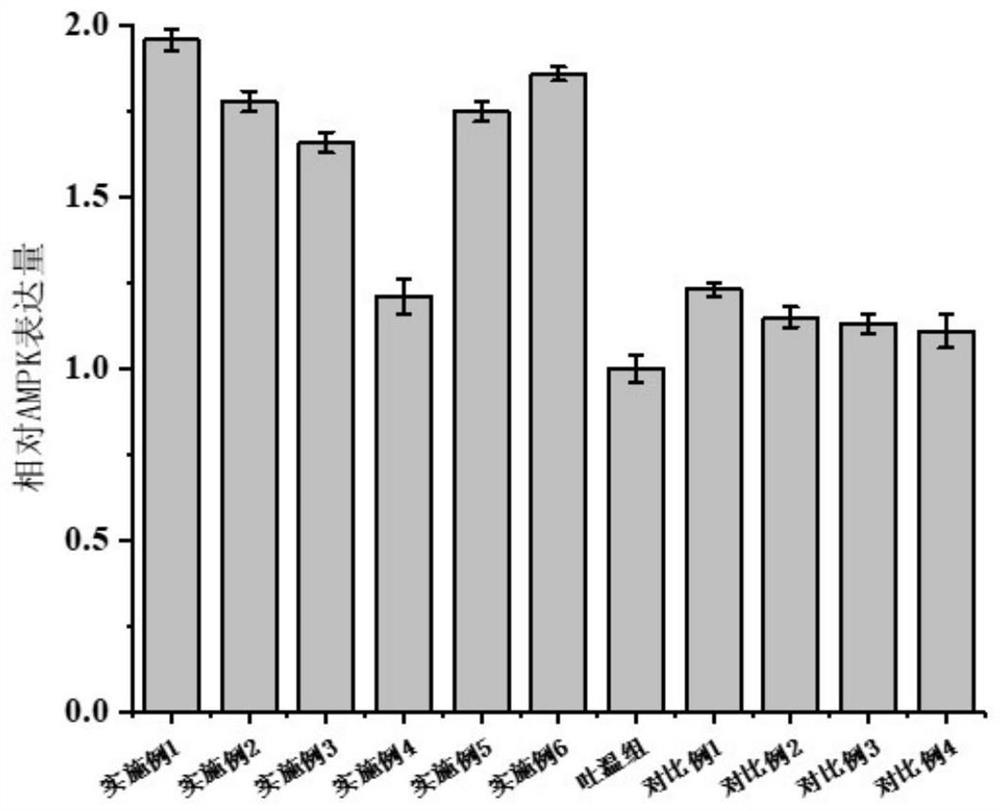
[0060] The expression of mTOR, AMPK, and NF-κB in muscle tissue, the fluidity of cell membrane, and the fatty acid composition of muscle cells were evaluated and analyzed through the aged zebrafish model. The results are as follows: figure 2 , image 3 , Figu...
Embodiment 3
[0062] Fat components used in sarcopenia medical formula foods, including the following raw materials in weight percentages: 60% medium chain triglycerides, 10% sesame oil, 10% flaxseed oil, 10% krill oil, 5% Fish oil, 5% peanut oil, each component is mixed and stirred with various oils and fats at room temperature to obtain a finished product; the specific preparation method is the same as in Example 1.
[0063] The fatty acid composition and lipid accompanying substances (sesaxanol, astaxanthin, tocopherol, etc.) content of the fat components were detected, and the results are shown in Table 1 and Table 2, respectively.
[0064] Muscle mass was assessed by the juvenile zebrafish model, as shown in figure 1 shown.
[0065] The expression of mTOR, AMPK, and NF-κB in muscle tissue, the fluidity of cell membrane, and the fatty acid composition of muscle cells were evaluated and analyzed through the aged zebrafish model. The results are as follows: figure 2 , image 3 , Fig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com