Oligomerization nucleic acid composition for preventing or treating canine distemper and application thereof
An oligonucleotide and DNA molecule technology, applied in the field of oligonucleotide combination for the prevention or treatment of canine distemper, can solve the problems of lax management, difficulty in general vaccines having broad applicability, and decreased vaccine antibody titers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] The preparation of the oligonucleotide of embodiment 1, modification
[0089] The sense strand and antisense strand shown in Table 1 were synthesized by Shanghai Gemma Pharmaceutical Technology Co., Ltd.
[0090] Dilute the sense strand with deionized water to obtain a sense strand dilution. Dilute the antisense strand with deionized water to obtain antisense strand dilution. Take the dilution solution of the sense strand and the corresponding dilution solution of the antisense strand, and perform annealing reaction to form oligonucleotides. The 3' end of each oligonucleotide is subjected to cholesterol modification, and the 5' end is subjected to phosphorylation modification to obtain a modified oligonucleotide.
[0091] A total of 28 oligonucleotides shown in Table 1 were prepared in this embodiment. A, G, C and U in each oligonucleotide represent adenine ribonucleotide, guanine ribonucleotide, cytosine ribonucleotide and uracil ribonucleotide in sequence, and T re...
Embodiment 2
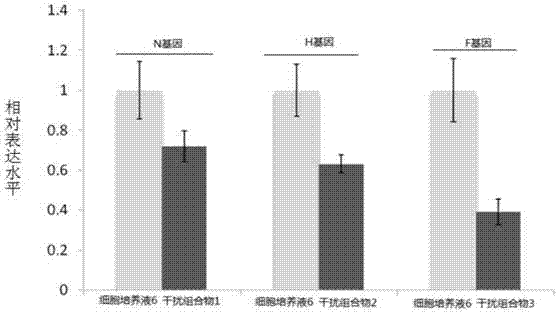
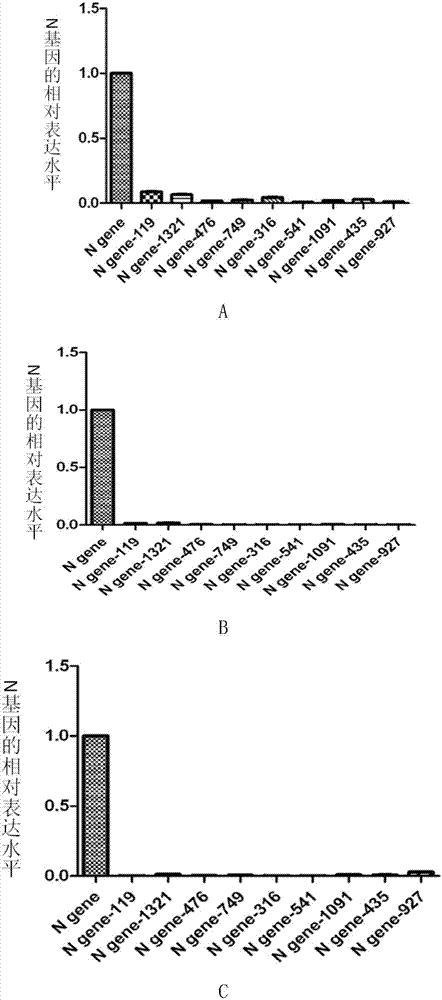
[0095] The modified oligonucleotide prepared by embodiment 2, embodiment 1 is on the impact of the relative expression of N gene, F gene and H gene of CDV
[0096] 1. Construction of recombinant plasmids
[0097] 1. Construction of recombinant plasmid pCDNA3.1-N
[0098] (1) According to the nucleotide sequence of the N gene of CDV (Sequence ID: gb|EU409838.1| or Genebank: EU409838.1), designed and synthesized by Shanghai Jima Pharmaceutical Technology Co., Ltd. as shown in sequence 49 in the sequence list DNA fragments. The 1st to 11th positions from the 5' end of sequence 49 are protective bases and the recognition sequence of restriction endonuclease HindⅢ, the 12th to 1583rd are the nucleotide sequence of the N gene, and the 1584th to 1594th are protective Base and recognition sequence of restriction endonuclease EcoRI.
[0099] (2) Digest the DNA fragment synthesized in step (1) with restriction endonuclease Hind III and EcoR I, and recover the digested product with a ...
Embodiment 3
[0155] The modified oligonucleotide prepared by embodiment 3 and embodiment 1 inhibits the replication of Vero cell virus infected with canine distemper attenuated CDV3-CL strain
[0156] 1. Preparation of Interfering Compositions
[0157] 1. Interfering composition 1
[0158] Take the N gene-119, N gene-476, N gene-749, N gene-435 and N gene-927 prepared in Example 1 and mix them according to the molar ratio of 1:1:1:1:1 to obtain the interference combination Object 1.
[0159] 2. Interference composition 2
[0160] F gene-485, F gene-685, F gene-792 and F gene-1279 prepared in Example 1 were mixed according to a molar ratio of 1:1:1:1 to obtain interference composition 2.
[0161] 3. Interference composition 3
[0162] Hgene-416, Hgene-947, Hgene-1373, Hgene-1447 and Hgene-1664 prepared in Example 1 were mixed according to the molar ratio of 1:1:1:1:1 to obtain interference composition 3.
[0163] 4. Interference composition 4
[0164] The H gene-119, F gene-485 and H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com