Cyclic peptide compound as well as preparation method and application thereof
A technology of compound and cyclic peptide, which is applied in the field of cyclic peptide compound and its preparation, can solve the problems of binding and breaking, and achieve the effect of rapid response and fast response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Determining the structure by taking Lys's own OPA cyclization as an example
[0091]
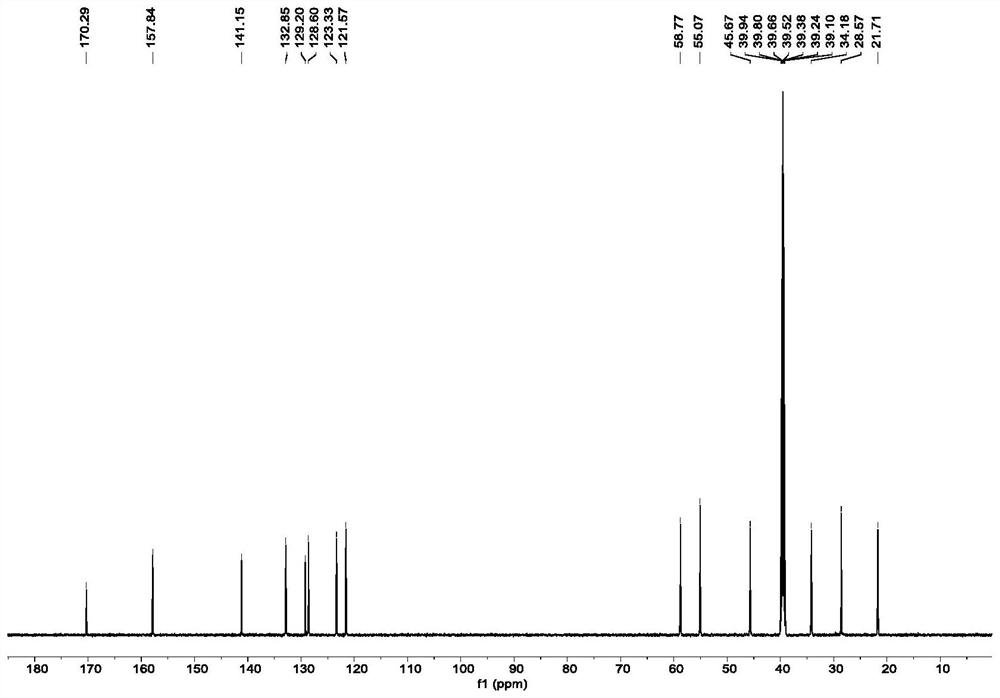
[0092] Lys (1.0equiv, 0.02mmol, 2.9mg) was dissolved in 2mL of PBS buffer (pH=8.0) and 2mL of MeOH at room temperature, and then 2.68mg (1.0equiv) of o-phthalaldehyde (OPA) was weighed and added to the solution, After stirring at room temperature for 5 min, the product of Lys self-cyclization was obtained. Purification and isolation were carried out by HPLC. Lys self-cyclization product 1 H-NMR, 13 C-NMR, DEPT-135, HSQC and NOESY spectra are listed in figure 1 .
[0093] 1 H NMR (600MHz, DMSO-d 6 )δ7.94(d,J=7.8Hz,1H),7.77-7.70(m,2H),7.63-7.60(m,1H),5.09–4.88(m,2H),4.51–4.40(m,2H) ,3.77–3.68(m,1H),2.35-2.26(m,1H),2.08-1.98(m,1H),1.94-1.85(m,1H),1.75-1.59(m,2H),1.49-1.38( m,1H). 13 C NMR (150MHz, DMSO-d 6 )δ 170.3, 157.8, 141.2, 132.9, 129.2, 128.6, 123.3, 121.6, 58.8, 55.1, 45.7, 34.2, 28.6, 21.7.
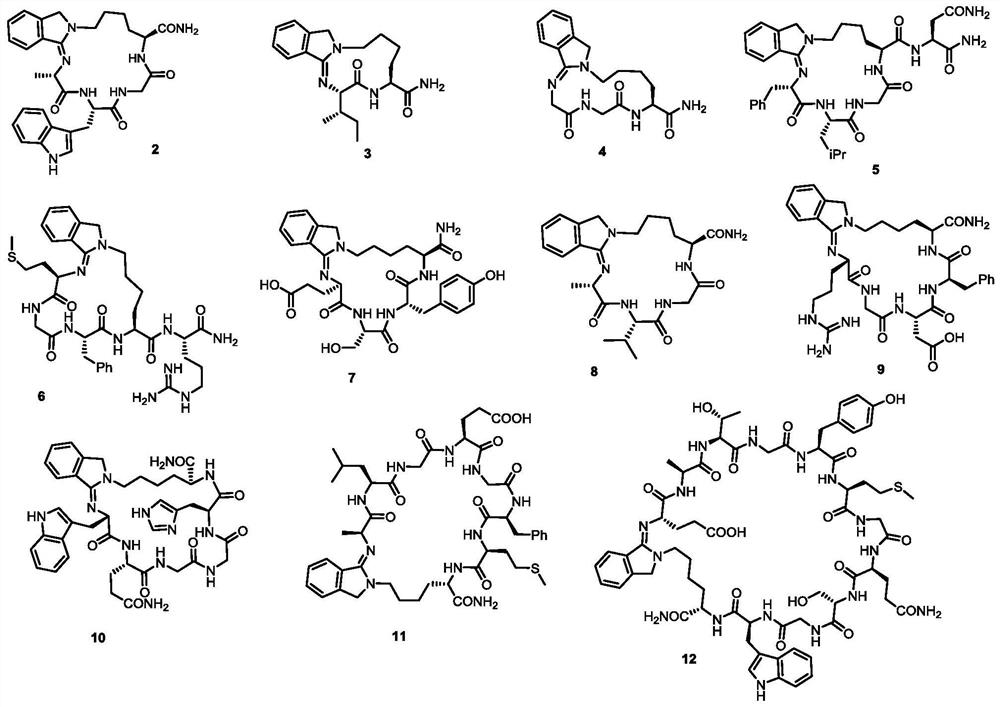
Embodiment 2-15
[0095] Taking Linear Peptide S2 as an Example to Introduce the Head-Side Chain Cyclization Process
[0096]
[0097] Condition [A]: Weigh the linear peptide (S2) (TFA salt, 0.02 mmol, 13.1 mg) into an 8 mL reaction vial, add 2 mL of PBS buffer (pH=8.0) and 2 mL of MeOH to completely dissolve the linear peptide. Subsequently, 2.68 mg (1.0 equiv) of o-phthalaldehyde was weighed and added into the solution, stirred for 5 min, and purified by HPLC to obtain a cyclization product.
[0098] Condition [B]: Weigh the linear peptide (S2) (TFA salt, 0.02mmol, 13.1mg) into an 8mL reaction vial, add 2mL H 2 O and 2 mL of MeOH were used to completely dissolve the linear peptide, followed by the addition of 3 equiv DIPEA to neutralize the TFA of the linear peptide. Subsequently, 2.68 mg (1.0 equiv) of o-phthalaldehyde was weighed and added into the solution, stirred for 5 min, and purified by HPLC to obtain a cyclization product.
[0099] Condition [C]: Weigh the linear peptide (S2) (T...
Embodiment 2
[0109] The impact of reaction concentration on reaction time in embodiment 2
[0110] Weigh the linear peptide S2 (TFA salt, 0.02mmol, 13.1mg) into an 8mL reaction bottle, add 2mL PBS buffer (pH=8.0) and 2mL MeOH to completely dissolve the linear peptide, and prepare a solution with a concentration of 5mM. Then weigh 2.68 mg (1.0 equiv) of o-phthalaldehyde (OPA) and add it into the solution. After stirring for 10 s, take out 50 ul of the reaction system and put it into a 1.5 ml EP tube, and add 5 ul of HCOOH to quench the reaction system. Monitored by LC-MS, the raw material can be completely reacted within 10s.
[0111] Similarly, the linear peptide S2 was reacted at a concentration of 50uM, and the raw material could be completely reacted within 2 minutes. Therefore, it can be explained that the reaction is very rapid, and the lower the reaction concentration, the longer the required reaction time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com