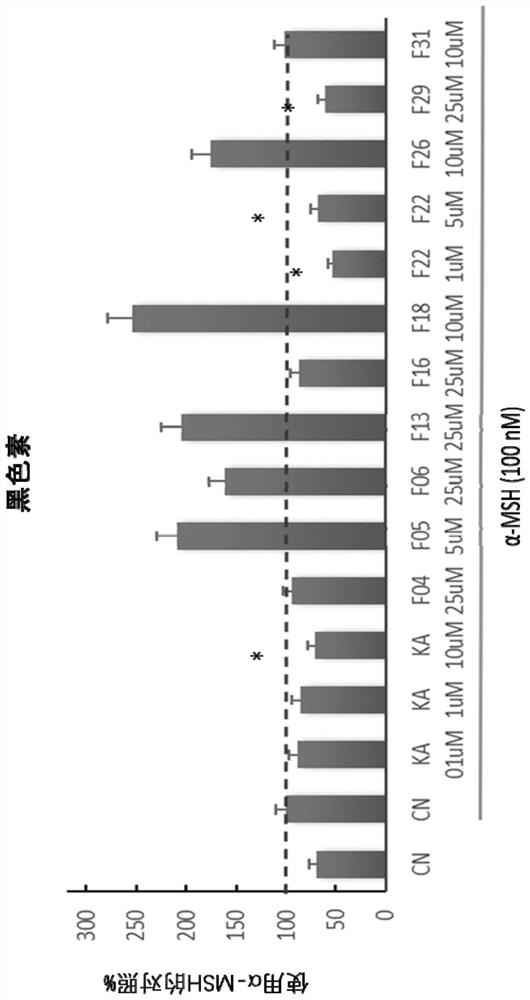
Melanocyte modulating peptides
A halogen, alkyl technology, used in the field of peptides that regulate melanin production, therapy and cosmetics, can solve the problem of no benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0159] The preparation method of the peptide according to the first aspect of the present invention comprises:
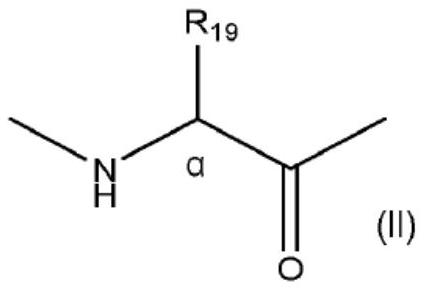
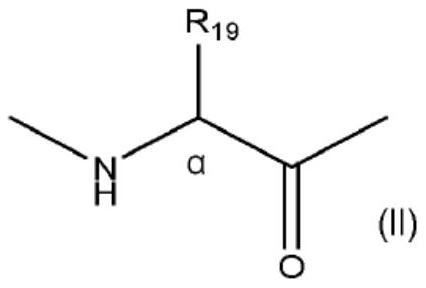
[0160] (1.a) Coupling the corresponding amino acid of the peptide with a compound of formula (III) and a compound of formula (IV) by condensation corresponding to the compounds designated as "i" and "i+4" or "i+7" amino acid. Compounds (III) and (IV) will undergo a subsequent cyclization step to generate the "L" diradical:
[0161]
[0162] where R 19 As defined above, Z 1 and Z 2 same or different, and represents (C 2 -C 10 ) alkenyl; and (1.b) a cyclization step comprising carrying out Z in solution with a Grubbs (I or II generation) catalyst 1 and Z 2 (see KimYoung-Woo et al., "Synthesis of all-hydrocarbon stapled a-helical peptides byring-closing olefin metathesis", Nature Protocols, 2011, 6(6), pp. 761-771; ScottJ.M. et al. Al, "Application of Ring-Closing Metathesis to the Synthesis of Rigidified Amino Acids and Peptides", J.Am.Chem.Soc., 1996, v.11...
Embodiment
[0220] 1. Materials and Methods
[0221] General procedure for synthesis
[0222] Compound IDP-F
[0223] Materials were purchased as follows: Fmoc-protected α-amino acid (---); Rinkamide MBHA resin (Tianjin Nankai Hecheng Technology Co., Ltd.); HBTU ((2-(1H-benzotriazol-1-yl)-1,1 ,3,3-Tetramethyluronium hexafluorophosphate), GLBiochem); N-methylmorpholine (Sinopharm Chemical Reagent Co., Ltd.); Succinic anhydride (Aladdin); Acetonitrile (Xingke Chemical); Ninhydrin (Sinopharm Chemical Reagent Co., Ltd.); piperidine (Vertellus); dimethylformamide, DMF (Zhejiang Jiangshan Chemical Co., Ltd.); trifluoroacetic acid, TFA (trifluoroacetic acid, Solvay), TIS (thioanisole, Solvay).
[0224] Briefly, linear peptides are artificially synthesized using Fmoc-based SPPS (solid-phase peptide synthesis) using Rink amide MBHA resin as a carrier.
[0225] The following scheme was used:
[0226] 1. Removal of the Fmoc protecting group with 20% piperidine in DMF.
[0227] 2. Wash the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com