Dried ginger quality evaluation method based on UPLC-TQ-MS/MS
A UPLC-TQ-MS and quality evaluation technology, which is applied in the field of quality analysis of traditional Chinese medicine, can solve the problems of single quantitative component type, long time for quality analysis of dried ginger, inability to effectively distinguish dried ginger and sulfur-smoked dried ginger, and achieve specificity Strong, guaranteed safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Treatment of Samples
[0033] 1, preparation of sample solution
[0034] Precisely weigh 0.1 g of dry ginger sample, extract 30 min with 10 ml 70% methanol (V / V), extract 13225 g from 10 min, to obtain a supernatant, diluted with 70% methanol (V / V) 100 μg / ml.
[0035] 2. Preparation of the standard solution of reference
[0036] Separating 6-gingerol, 6-gingerol, 6-dehydrogenol, 8-gingerol, 8-ginger, 8-dehydrotanol, 8-ginger, 8-dehydrogenol, 10 Ginger, 10-ginger, 10-dehydrotanculid, and spectroscopy of nuclear magnetic and high resolution mass spectrometry, the peak area is collected by the peak area, and the purity is higher than 98%.
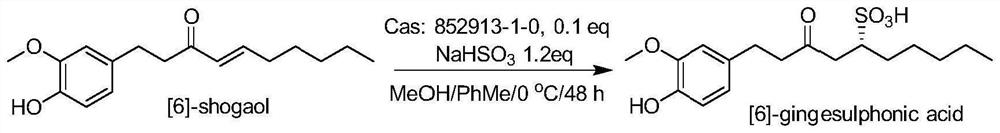
[0037] 6-gingerol (160 mg) was dissolved in MeOH / PHME solution (4.5 mL / 1.5 ml), and add catalyst at 0 ° C (C 29 Hide 30 N 4O Fly 6 S, CAS: 852913-19-0, 35 mg, 0.1 equivalents) and NAHSO 3Aqueous solution (0.5m, 72 mg). After stirring at 0 ° C for 48 hours, the reaction was stopped and dried, then dissolved in methanol, f...
Embodiment 2
[0039] Example 2 Chromatography Condition Optimization
[0040] UPLC analysis is carried out on the ACQUITY UPLC 1-Class system equipped with a binary solvent system. Chromatography separation was achieved under 40 ° C column temperature conditions using a BEH SHIELD RP18 column. The mobile phase consists of water (a) and acetonitrile (B), both comprising 0.1% formic acid (V / V), gradient elution at a flow rate of 0.4 ml / min, and the injection volume was set to 2 μL.
[0041] By examining different elution procedures, the gradient elution process that can make the compound has better separation and better peak time as follows: 5% B, 0-0.5 min; 5-80% B, 0.5-10 min 80-100% B, 10-12 min; 100% B, 12-13 min, 100-5% B, 13-14 min; 5% B, 14-15 min.
[0042] The UPLC system and the triple quadrupole mass spectrometer are: using an electrospray ion source (ESL), a capillary voltage 2 kV, a tapered pore voltage 20 V, a dehydric gas temperature of 250 ° C, cone The pore gas flow rate is 15...
Embodiment 3
[0044] Example 3 Construction of Quantitative Analysis Method
[0045] 1, specialty test
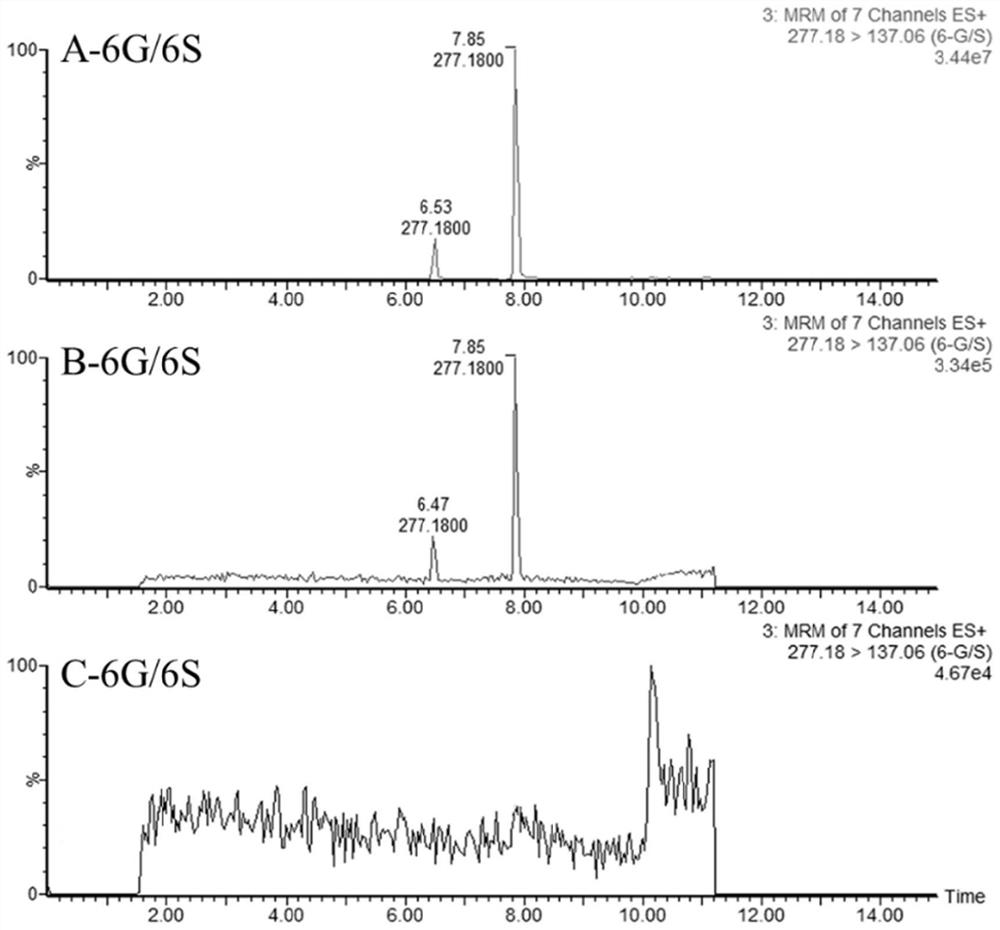
[0046] The dry ginger sample solution obtained by Example 1 was applied, and the standard solution and blank solvent were performed, and UPLC-TQ-MS / MS measurement analysis was performed in accordance with the chromatographic conditions in Example 2. Figure 2-8 Indicated. Among them, A, B, and C represent dry ginger samples, standard solutions, and white solvents. 6GS, 6G, 6S, 6D, 8G, 8S, 8D, 10G, 10S and 10D represent 6-gumullic acid (retention time = 6.5 min), 6-gingerol (retention time = 6.5 min), 6-ginger Phenol (retention time = 7.8 min), 6-dehydrogenin (retention time = 8.7 min), 8-gingerol (retention time = 7.6 min), 8-gingerol (retention time = 8.9 min), 8 - Dehydrotionin (retention time = 9.6 min), 10-gingerol (retention time = 8.6 min), 10-gingerol (retention time = 9.8 min) and 10-dehydrotanolidone (retention time = 10.5 min).
[0047] According to this result, the sample matrix ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com