Method for evaluating likelihood of onset or progression of transplanted kidney chronic rejection and chronic kidney disease, test kit, and pharmaceutical composition
A technology for chronic kidney disease and chronic rejection, applied in drug combinations, biochemical equipment and methods, allergic diseases, etc., can solve problems such as the functional role and expression level of SYT17 protein that have not yet been found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0110] experimental method
[0111] 1) Patient and urine sample
[0112] With permission from the Ethics Committee of Hokkaido University Hospital, total urine was obtained from healthy volunteers, chronic kidney disease (CKD) patients, and kidney transplant (KTx) patients between 2016 and 2019. Urine collection for KTx patients was performed at the time of procedural biopsy, excluding urine at the time of needle (episode) biopsy. Urine was centrifuged at 1,500 rpm for 5 minutes, and the supernatant was collected and stored at -80°C.
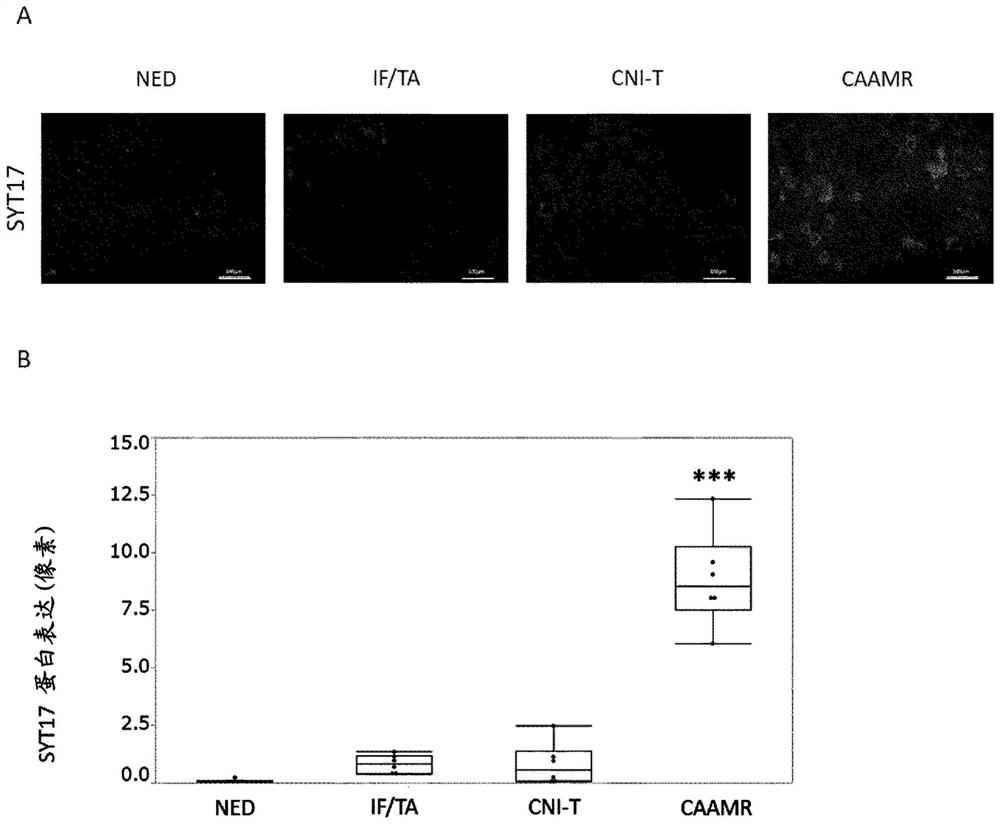
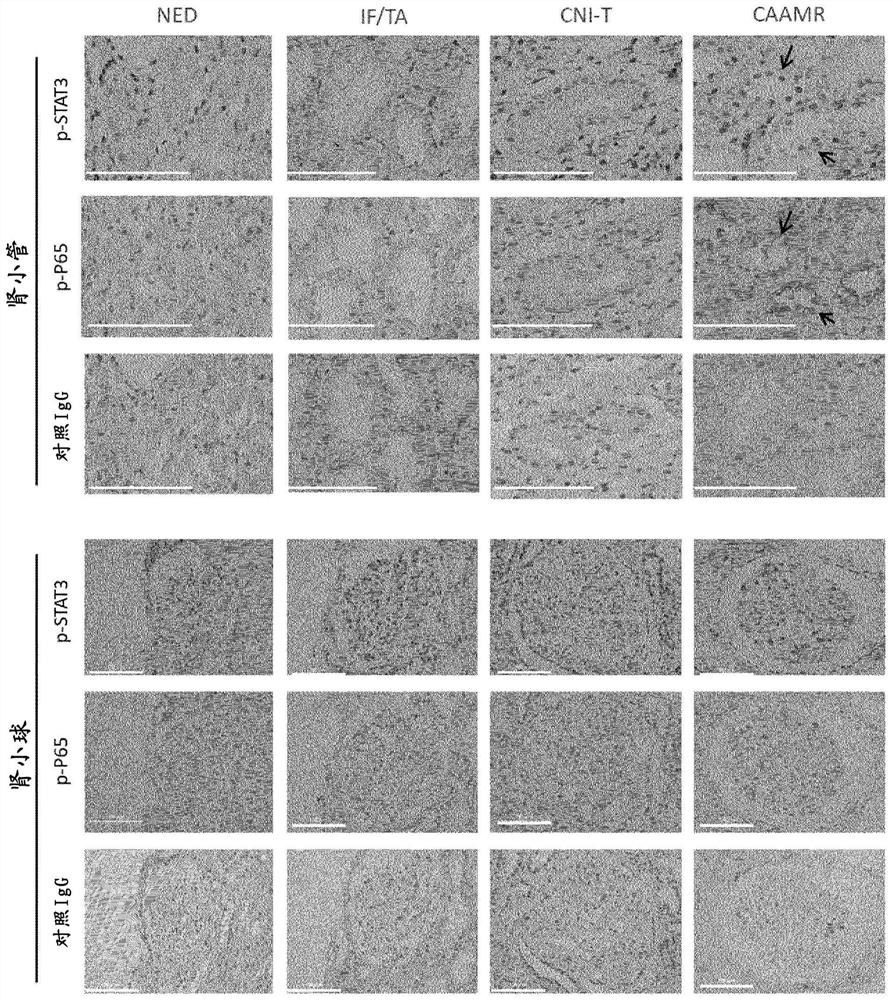
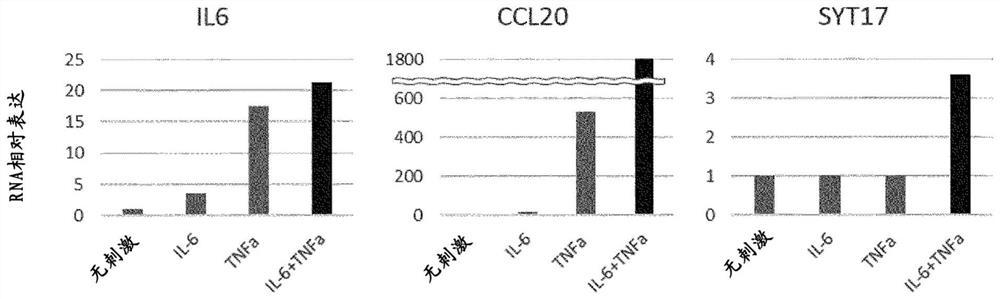
[0113] It should be noted that all KTx patients received standard immunosuppressive therapy of prednisone, mycophenolic acid, tacrolimus or cyclosporine. In addition, according to the histological results of procedural biopsy, KTx patients were divided into normal histology group (NED, n=20) and fibrosis group (interstitial fibrosis and interstitial fibrosis) based on Banff classification (2017). atrophy), IF / TA, n=19), calcineurin toxicity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com