Construction method and application of novel attenuated listeria monocytogenes based on amino acid modification
A Listeria and amino acid technology, applied in the field of genetic engineering, can solve the problems of low antigen presentation efficiency, insufficient body safety, low immunogenicity, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The construction of embodiment 1 attenuated vaccine vector
[0042] 1. Construction of recombinant plasmids
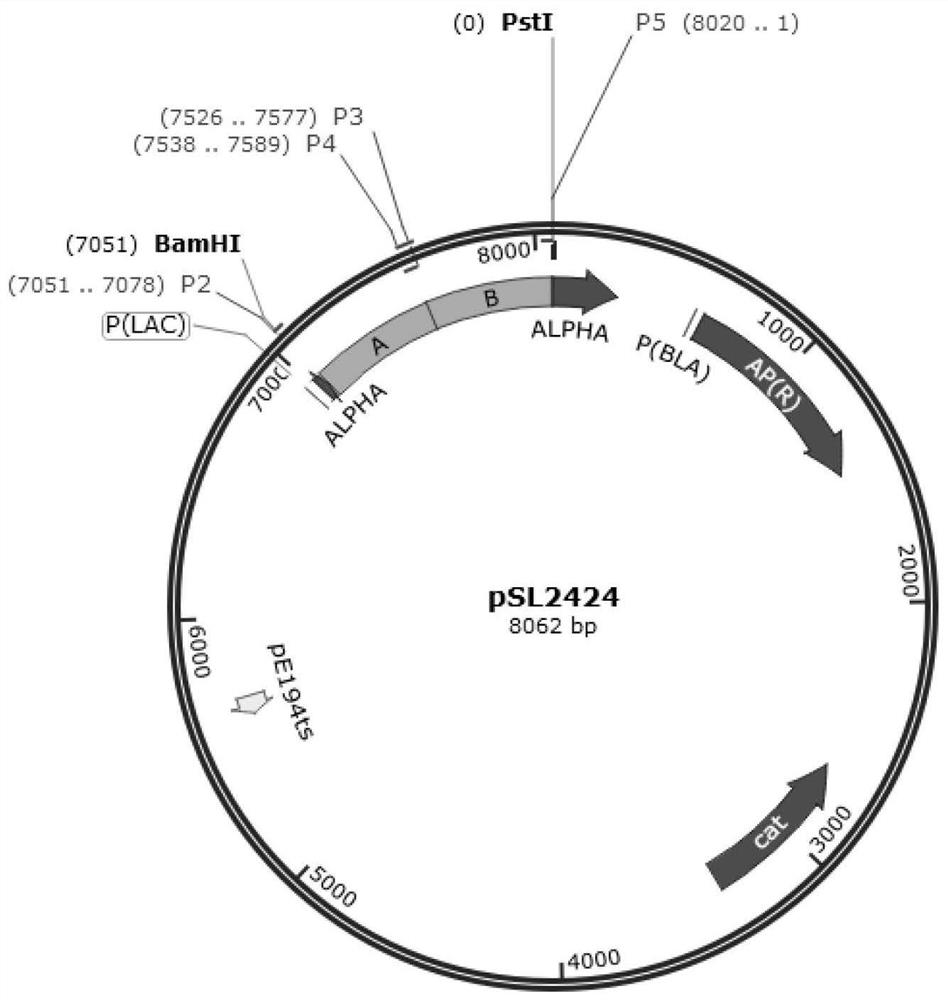
[0043]Using the Listeria monocytogenes standard strain genome (GenBank sequence number is NC_003210.1) as a template, use Snapgene software to select amino acids 87-250 in the hly gene as the upstream homology arm, and select amino acids 256-422 in the hly gene amino acid as the downstream homology arm. Fragment A (501 bp, amplification primers are P2 and P3) and fragment B (499 bp, amplification primers are P4 and P5) respectively containing restriction sites BamH I and Pst I were amplified by PCR. On this basis, the target fragment of "A-B" (as shown in SEQ ID NO.1) was amplified by overlapping PCR technique (SOE-PCR). Using BamH I and Pst I as restriction sites, the recombinant plasmid pSL2424 was obtained by restriction enzyme digestion and enzyme ligation to the Listeria shuttle plasmid pKSV7, which was stored at -20°C after sequencing verification.
[0...
Embodiment 2
[0055] Example 2 Attenuated strain infection biological analysis
[0056] 1. Mouse organ proliferation test
[0057] Listeria monocytogenes was infected by intraperitoneal injection in 18-22g ICR female mice (infection amount was about 10 6 CFU), the mouse liver and spleen were isolated after 24 and 48 hours of infection, respectively, added 10mM PBS (pH 7.4) to fully grind, then diluted to an appropriate concentration, and placed on the plate and cultured at 37°C for 24 hours, then counted the bacterial colonies. The results were as follows: log 10 CFU presented. Such as figure 2 As shown, compared with WT, the colonization ability of LAPD5155 in mouse liver (Liver) and spleen (Spleen) was significantly decreased.
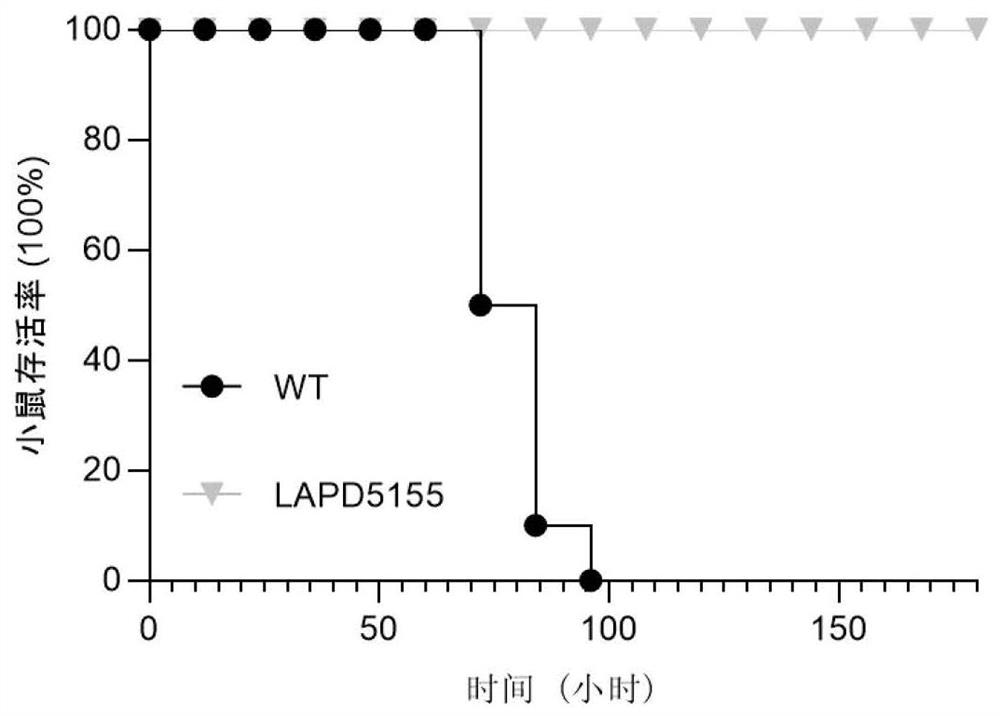
[0058] 2. Comparison of median lethal dose
[0059] Listeria monocytogenes was infected by intraperitoneal injection in 18-22g ICR female mice (infection amount was about 10 6 CFU), from the day of bacterial infection, observe the survival situation of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com